Magnesium Bicarbonate Formula . Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. It is formed by the action of water containing free carbon dioxide on.
from www.slideserve.com
Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. It is formed by the action of water containing free carbon dioxide on. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to.
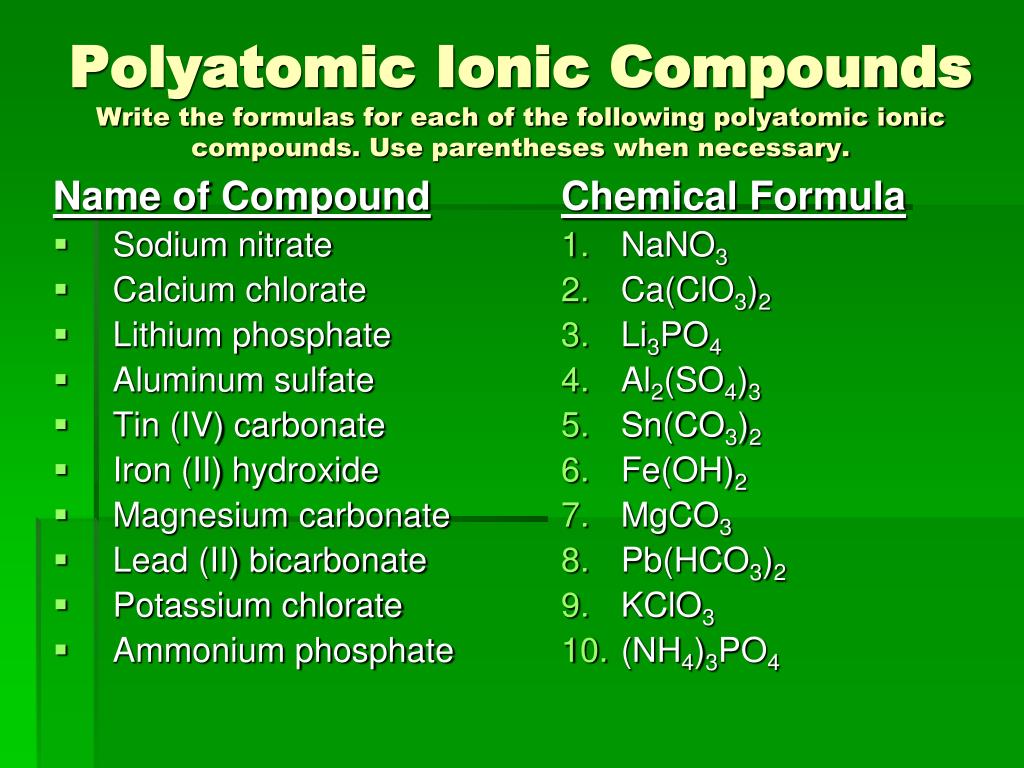
PPT Writing Ionic Formulas PowerPoint Presentation, free download
Magnesium Bicarbonate Formula Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. It is formed by the action of water containing free carbon dioxide on. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6.
From www.alamy.com
3D image of Magnesium bicarbonate skeletal formula molecular chemical Magnesium Bicarbonate Formula Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. It is formed by the action of water containing free carbon dioxide on. Mg2+ + 2 hco3− →. Magnesium Bicarbonate Formula.
From brainly.in
write a chemical formula of the following compound using criss cross Magnesium Bicarbonate Formula Mg2+ + 2 hco3− → mgco3 + co2 + h2o. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Lorsqu’il. Magnesium Bicarbonate Formula.
From www.chemistrylearner.com
Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses Magnesium Bicarbonate Formula Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. It has various names. Magnesium Bicarbonate Formula.
From www.pinterest.com
How to Make Magnesium Bicarbonate Water Natural Health by Karen Magnesium Bicarbonate Formula It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with. Magnesium Bicarbonate Formula.
From buy-pharm.com
Buy magnesium bicarbonate Online magnesium bicarbonate Magnesium Bicarbonate Formula Mg2+ + 2 hco3− → mgco3 + co2 + h2o. It is formed by the action of water containing free carbon dioxide on. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Magnesium bicarbonate is a salt with the formula c 2 h 2. Magnesium Bicarbonate Formula.
From www.youtube.com
DIY Magnesium Bicarbonate Supplement YouTube Magnesium Bicarbonate Formula It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. It is formed by the action of. Magnesium Bicarbonate Formula.
From www.youtube.com
How to Write the Formula for Magnesium bicarbonate (Magnesium hydrogen Magnesium Bicarbonate Formula It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. It is formed by the action of water containing free carbon dioxide on. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Magnesium bicarbonate is a salt. Magnesium Bicarbonate Formula.
From www.biocoherence.eu
Magnesium bicarbonate Biocoherence Nederland Magnesium Bicarbonate Formula Mg2+ + 2 hco3− → mgco3 + co2 + h2o. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium. Magnesium Bicarbonate Formula.
From www.youtube.com
Magnesium Bicarbonate Water (How I Make It) YouTube Magnesium Bicarbonate Formula It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is a. Magnesium Bicarbonate Formula.
From www.pinterest.com
pH Adjust Alkalinizing Formula Alkalinize with Potassium and Sodium Magnesium Bicarbonate Formula Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It has various names and. Magnesium Bicarbonate Formula.
From www.vedantu.com
Magnesium Bicarbonate Learn Important Terms and Concepts Magnesium Bicarbonate Formula Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco. Magnesium Bicarbonate Formula.
From worldhealthlife.com
Magnesium Bicarbonate Benefits, Side Effects, And More Magnesium Bicarbonate Formula Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo). Magnesium Bicarbonate Formula.
From www.youtube.com
How to Balance MgCO3 = MgO + CO2 of Magnesium carbonate Magnesium Bicarbonate Formula Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. It is formed by the action of water containing free carbon dioxide on. It can be prepared by treating magnesium acetate with sodium bicarbonate or by. Magnesium Bicarbonate Formula.
From healthbrake.com
5 Benefits Of Magnesium Bicarbonate Health Brake Magnesium Bicarbonate Formula Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. It is formed by the action of water containing free carbon dioxide on. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate,. Magnesium Bicarbonate Formula.
From www.alamy.com
3D image of Magnesium carbonate skeletal formula molecular chemical Magnesium Bicarbonate Formula Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. It is formed by the action of water containing free carbon dioxide on. Magnesium bicarbonate is a chemical compound with. Magnesium Bicarbonate Formula.
From www.shutterstock.com
23 Magnesium Carbonate Formula Images, Stock Photos & Vectors Magnesium Bicarbonate Formula Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It has various names. Magnesium Bicarbonate Formula.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Magnesium Bicarbonate Formula It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Magnesium bicarbonate. Magnesium Bicarbonate Formula.
From www.pinterest.com
How to Make Magnesium Bicarbonate Water Natural Health by Karen Magnesium Bicarbonate Formula It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo). Magnesium Bicarbonate Formula.
From www.livevitae.com
Magnesium Bicarbonate Powder (One Nutrition) 20 OFF Live Vitae Magnesium Bicarbonate Formula Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2). Magnesium Bicarbonate Formula.
From labquiz.netlify.app
Magnesium bicarbonate chemical formula labquiz Magnesium Bicarbonate Formula Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It is formed by the action of water containing free carbon dioxide on. Magnesium bicarbonate can be formed. Magnesium Bicarbonate Formula.
From www.youtube.com
Magnesiumcarbonat YouTube Magnesium Bicarbonate Formula Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon. Magnesium Bicarbonate Formula.
From www.aetherforce.energy
Magnesium BiCarbonate Aether Force Magnesium Bicarbonate Formula It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3). Magnesium Bicarbonate Formula.
From www.youtube.com
Molar Mass / Molecular Weight of MgCO3 Magnesium carbonate YouTube Magnesium Bicarbonate Formula Mg2+ + 2 hco3− → mgco3 + co2 + h2o. It is formed by the action of water containing free carbon dioxide on. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and.. Magnesium Bicarbonate Formula.
From www.pinterest.com
How to make a potent Magnesium Bicarbonate solution Magnesium water Magnesium Bicarbonate Formula It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de. Magnesium Bicarbonate Formula.
From brainly.in
Chemical formula of magnesium bicarbonate using criss cross method Magnesium Bicarbonate Formula Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. It is formed by the action of water containing free carbon dioxide on. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts. Magnesium Bicarbonate Formula.
From labquiz.netlify.app
Magnesium bicarbonate chemical formula labquiz Magnesium Bicarbonate Formula Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It can be prepared. Magnesium Bicarbonate Formula.
From hyvida.com
Magnesium Bicarbonate Why is it so impactful? HyVIDA Magnesium Bicarbonate Formula It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Magnesium bicarbonate is a salt. Magnesium Bicarbonate Formula.
From www.alamy.com
3D image of Magnesium glycinate skeletal formula molecular chemical Magnesium Bicarbonate Formula Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. It is formed by the action of water containing free carbon dioxide on. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de. Magnesium Bicarbonate Formula.
From www.youtube.com
How to write the formula for magnesium bicarbonate YouTube Magnesium Bicarbonate Formula Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du. Magnesium Bicarbonate Formula.
From www.freepng.fr
Carbonate De Magnésium, Carbonate De, Le Magnésium PNG Carbonate De Magnesium Bicarbonate Formula Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It is formed by the action of water containing free carbon dioxide on. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6.. Magnesium Bicarbonate Formula.
From www.fishersci.ch
Magnesiumcarbonat, für Biochemie, USP, Thermo Scientific Fisher Magnesium Bicarbonate Formula Magnesium bicarbonate is a chemical compound with the formula c2h2mgo6. It is formed by the action of water containing free carbon dioxide on. Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide. Magnesium Bicarbonate Formula.
From labquiz.netlify.app
Magnesium bicarbonate chemical formula labquiz Magnesium Bicarbonate Formula It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. It is formed by the action of water containing free carbon dioxide on. It has various names and. Magnesium Bicarbonate Formula.
From janicerwesto.blob.core.windows.net
Magnesium Bicarbonate Function at janicerwesto blog Magnesium Bicarbonate Formula Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. Lorsqu’il est exposé à un environnement acide, le bicarbonate de magnésium se décompose pour produire de l’oxyde de magnésium, du dioxyde de. It can be prepared by treating magnesium acetate with sodium bicarbonate or by reacting magnesium hydroxide with carbon dioxide. Magnesium bicarbonate is a chemical compound with the. Magnesium Bicarbonate Formula.
From www.britannica.com
magnesium Description, Properties, & Compounds Britannica Magnesium Bicarbonate Formula Magnesium bicarbonate is a salt with the formula c 2 h 2 mgo 6 or mg (hco 3) 2. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. It is formed by the action of water containing free carbon dioxide on. Mg2+ +. Magnesium Bicarbonate Formula.
From www.numerade.com
SOLVED Cation Anion Formula Name Mg2+ Bicarbonate Mg(HCO3)2 Magnesium Magnesium Bicarbonate Formula Mg2+ + 2 hco3− → mgco3 + co2 + h2o. Magnesium bicarbonate is an unstable compound with the formula mg (hco3)2. It has various names and synonyms, such as carbonic acid, magnesium salt, magnesium dibicarbonate, and. Magnesium bicarbonate can be formed when magnesium hydroxide (mg (oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to. Magnesium. Magnesium Bicarbonate Formula.