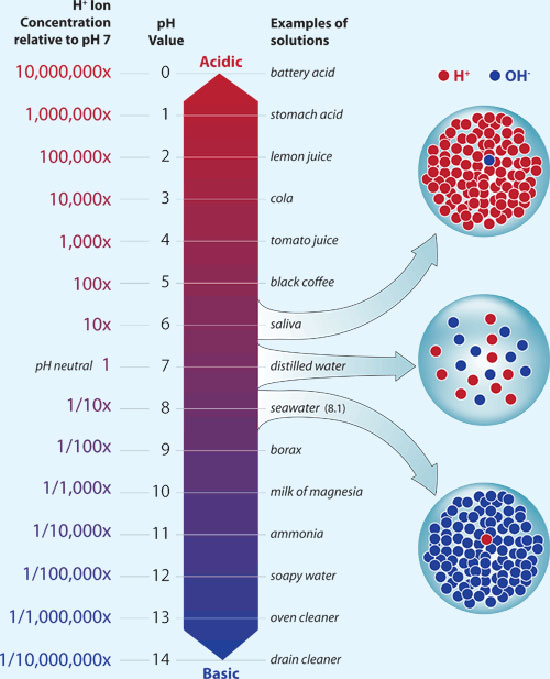
What Is The Relationship Between Ph And H+ . The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that represents the concentration of hydrogen ions in a solution. This means that each value is 10 times the value below it. The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The ph of a solution is the negative logarithm (base 10) of the hydrogen. For example, ph 5 is 10. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. The ph scale is a logarithmic scale with base 10; Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. What a person measures in the. What is the relationship between ph and hydrogen ion concentration?
from socratic.org
Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. This means that each value is 10 times the value below it. What is the relationship between ph and hydrogen ion concentration? For example, ph 5 is 10. The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The ph scale is a logarithmic scale with base 10; The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that represents the concentration of hydrogen ions in a solution. The ph of a solution is the negative logarithm (base 10) of the hydrogen. What a person measures in the.
What is difference between a pH of 8 and a pH of 12 in terms of H+
What Is The Relationship Between Ph And H+ The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. What a person measures in the. For example, ph 5 is 10. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. What is the relationship between ph and hydrogen ion concentration? The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The ph of a solution is the negative logarithm (base 10) of the hydrogen. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that represents the concentration of hydrogen ions in a solution. The ph scale is a logarithmic scale with base 10; This means that each value is 10 times the value below it. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge.
From www.youtube.com
Lecture 111320 The relationship between pH, pOH, [H+], and [OH What Is The Relationship Between Ph And H+ The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. What is the relationship between ph and hydrogen ion concentration? The ph of a solution is the negative logarithm (base 10) of the hydrogen. What a person measures in the. This means that each value is 10 times the value below it. The. What Is The Relationship Between Ph And H+.
From socratic.org
What is difference between a pH of 8 and a pH of 12 in terms of H+ What Is The Relationship Between Ph And H+ The ph of a solution is the negative logarithm (base 10) of the hydrogen. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. This means that each value is 10 times the value below it. The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. What is the. What Is The Relationship Between Ph And H+.
From www.youtube.com
The relationship between pH and Hydrogen ion [H+]. YouTube What Is The Relationship Between Ph And H+ This means that each value is 10 times the value below it. What is the relationship between ph and hydrogen ion concentration? The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is. What Is The Relationship Between Ph And H+.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel What Is The Relationship Between Ph And H+ What a person measures in the. Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. The ph scale is a logarithmic scale with base 10; For example, ph 5 is 10. This means that each value is 10 times the value below it. The molar concentration of dissolved hydrogen ions in solution. What Is The Relationship Between Ph And H+.
From www.numerade.com
SOLVED Relationship between pH and pOH. Remember to fill in the table What Is The Relationship Between Ph And H+ For example, ph 5 is 10. The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h. What Is The Relationship Between Ph And H+.
From www.numerade.com
SOLVED the relationship between pH and hydrogen ion concentration in What Is The Relationship Between Ph And H+ The ph of a solution is the negative logarithm (base 10) of the hydrogen. What is the relationship between ph and hydrogen ion concentration? The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. What a person measures in. What Is The Relationship Between Ph And H+.
From netsolwater.com
What is the relationship between pH and alkalinity What Is The Relationship Between Ph And H+ The ph of a solution is the negative logarithm (base 10) of the hydrogen. The ph scale is a logarithmic scale with base 10; What is the relationship between ph and hydrogen ion concentration? The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. What. What Is The Relationship Between Ph And H+.
From studylibraryfraiche.z14.web.core.windows.net
Ph And Poh Explained What Is The Relationship Between Ph And H+ What is the relationship between ph and hydrogen ion concentration? The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that represents the concentration of hydrogen ions in a solution. Acidic and alkaline solutions can conduct. What Is The Relationship Between Ph And H+.
From www.w3schools.blog
Concept of pH W3schools What Is The Relationship Between Ph And H+ Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. For example, ph 5 is 10. The ph scale is a logarithmic scale with base 10; The molar. What Is The Relationship Between Ph And H+.
From www.expii.com
What Is pH? — Definition & Overview Expii What Is The Relationship Between Ph And H+ The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that. What Is The Relationship Between Ph And H+.
From www.slideserve.com
PPT pKa concepts PowerPoint Presentation, free download ID6594110 What Is The Relationship Between Ph And H+ The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that represents the concentration of hydrogen ions in a solution. What a person measures in the. What is the relationship between ph and hydrogen ion concentration? This means that each value is 10 times the value below it. The ph scale is a. What Is The Relationship Between Ph And H+.
From mungfali.com
Hydrogen Ions And PH What Is The Relationship Between Ph And H+ For example, ph 5 is 10. What is the relationship between ph and hydrogen ion concentration? The ph scale is a logarithmic scale with base 10; The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The main difference between both scales is that in thermodynamic ph scale one is interested not in. What Is The Relationship Between Ph And H+.
From www.youtube.com
Relationship between pH and [H+] YouTube What Is The Relationship Between Ph And H+ For example, ph 5 is 10. The ph of a solution is the negative logarithm (base 10) of the hydrogen. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. This means that each value is 10 times the. What Is The Relationship Between Ph And H+.
From www.slideserve.com
PPT Acids and Bases 9 / 03 / 2009 PowerPoint Presentation, free What Is The Relationship Between Ph And H+ The ph of a solution is the negative logarithm (base 10) of the hydrogen. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit. What Is The Relationship Between Ph And H+.
From www.youtube.com
acid base chemistry relation between pH pOH [H] [OH] YouTube What Is The Relationship Between Ph And H+ For example, ph 5 is 10. This means that each value is 10 times the value below it. What is the relationship between ph and hydrogen ion concentration? The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. The term “ph” is an abbreviation for. What Is The Relationship Between Ph And H+.
From socratic.org
How to you convert between pH, pOH, [H+] and [OH]? Socratic What Is The Relationship Between Ph And H+ The ph scale is a logarithmic scale with base 10; The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The ph of a solution is the negative logarithm (base 10) of the hydrogen. The main difference between both. What Is The Relationship Between Ph And H+.
From www.numerade.com
SOLVEDAnswer the following questions about ionic equilibrium of water What Is The Relationship Between Ph And H+ The ph scale is a logarithmic scale with base 10; What is the relationship between ph and hydrogen ion concentration? The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h. What Is The Relationship Between Ph And H+.
From www.thinkswap.com
Experiment The Relationship between Hydrogen Ions, H+ or Hydroxide What Is The Relationship Between Ph And H+ What is the relationship between ph and hydrogen ion concentration? The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The ph scale is a logarithmic scale with base 10; The. What Is The Relationship Between Ph And H+.
From www.shutterstock.com
Ph Scale Chart Corresponding Hydrogen Ion Stock Vector 293946068 What Is The Relationship Between Ph And H+ This means that each value is 10 times the value below it. The ph scale is a logarithmic scale with base 10; The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. What a person measures in the. The main difference between both scales is that in thermodynamic ph scale one is interested. What Is The Relationship Between Ph And H+.
From www.slideserve.com
PPT BCHS 3304 General Biochemistry I, Section 07553 Spring 2003 100 What Is The Relationship Between Ph And H+ The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. For example, ph 5 is 10. What a person measures in the. The ph scale is a logarithmic. What Is The Relationship Between Ph And H+.
From slideplayer.com
PH and pOH Chem ppt download What Is The Relationship Between Ph And H+ This means that each value is 10 times the value below it. What is the relationship between ph and hydrogen ion concentration? The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The ph of a solution is the negative logarithm (base 10) of the hydrogen. What a person measures in the. The ph scale is. What Is The Relationship Between Ph And H+.
From www.youtube.com
Introduction to pH and pOH // HSC Chemistry YouTube What Is The Relationship Between Ph And H+ What is the relationship between ph and hydrogen ion concentration? This means that each value is 10 times the value below it. Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. The ph scale is a logarithmic scale with base 10; The ph of a solution is the negative logarithm (base 10). What Is The Relationship Between Ph And H+.
From www.slideserve.com
PPT Acids, Bases, and Indicators PowerPoint Presentation, free What Is The Relationship Between Ph And H+ The ph scale is a logarithmic scale with base 10; For example, ph 5 is 10. What is the relationship between ph and hydrogen ion concentration? The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that represents the concentration of hydrogen ions in a solution. This means that each value is 10. What Is The Relationship Between Ph And H+.
From general.chemistrysteps.com
pH and pKa Relationship Chemistry Steps What Is The Relationship Between Ph And H+ The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. For example, ph 5 is 10. Acidic and alkaline solutions can conduct electricity because they have ions that. What Is The Relationship Between Ph And H+.
From www.slideserve.com
PPT AcidBase Chemistry PowerPoint Presentation, free download ID What Is The Relationship Between Ph And H+ Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. The ph scale is a logarithmic scale with base 10; This means that each value is 10 times the value below it. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but. What Is The Relationship Between Ph And H+.
From slideplayer.com
Hydrogen Ions and Acidity ppt download What Is The Relationship Between Ph And H+ The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. For example, ph 5 is 10. This means that each value is 10 times the value below it. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity.. What Is The Relationship Between Ph And H+.
From www.youtube.com
Relationships between pH, pOH, and pKw (Pt 3) YouTube What Is The Relationship Between Ph And H+ The ph scale is a logarithmic scale with base 10; The ph of a solution is the negative logarithm (base 10) of the hydrogen. What a person measures in the. For example, ph 5 is 10. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. What is the relationship between ph and hydrogen ion concentration?. What Is The Relationship Between Ph And H+.
From www.slideserve.com
PPT Calculating pH and pOH PowerPoint Presentation, free download What Is The Relationship Between Ph And H+ For example, ph 5 is 10. The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. This means that each value is 10 times the value below it. Acidic and alkaline solutions can conduct electricity because they have ions. What Is The Relationship Between Ph And H+.
From www.numerade.com
SOLVED the relationship between pH and hydrogen ion concentration in What Is The Relationship Between Ph And H+ The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. What is the relationship between ph and hydrogen ion concentration? The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. The ph scale is a logarithmic scale with base 10; The. What Is The Relationship Between Ph And H+.
From www.slideshare.net
Lecture 19.2 pH What Is The Relationship Between Ph And H+ Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. The ph of a solution is the negative logarithm (base 10) of the hydrogen. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that represents the concentration of hydrogen ions in a solution. The ph. What Is The Relationship Between Ph And H+.
From slideplayer.com
ACID BASE DISTURBANCES and INTERPRETING ABG ppt download What Is The Relationship Between Ph And H+ The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. What is the relationship between ph and hydrogen ion concentration? The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The term “ph” is an abbreviation for the “potential of hydrogen.” ph is a unit of measurement that represents. What Is The Relationship Between Ph And H+.
From pediaa.com
Relationship Between Hydrogen Ions and pH Definition, Properties, pH What Is The Relationship Between Ph And H+ This means that each value is 10 times the value below it. Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. What a person measures in the.. What Is The Relationship Between Ph And H+.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 What Is The Relationship Between Ph And H+ The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The main difference between both scales is that in thermodynamic ph scale one is interested not in h + concentration, but in h + activity. This means that each value is 10 times the value below it. For example, ph 5 is 10. The term “ph”. What Is The Relationship Between Ph And H+.
From slideplayer.com
Chemical Equilibrium and pH ppt download What Is The Relationship Between Ph And H+ Acidic and alkaline solutions can conduct electricity because they have ions that are free to carry charge. This means that each value is 10 times the value below it. For example, ph 5 is 10. What is the relationship between ph and hydrogen ion concentration? The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The. What Is The Relationship Between Ph And H+.
From saylordotorg.github.io
Aqueous AcidBase Equilibriums What Is The Relationship Between Ph And H+ The flowchart shows the way to convert between hydrogen ion concentration, hydroxide ion concentration, ph, and poh. The ph scale is a logarithmic scale with base 10; This means that each value is 10 times the value below it. The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. The term “ph” is an abbreviation for. What Is The Relationship Between Ph And H+.