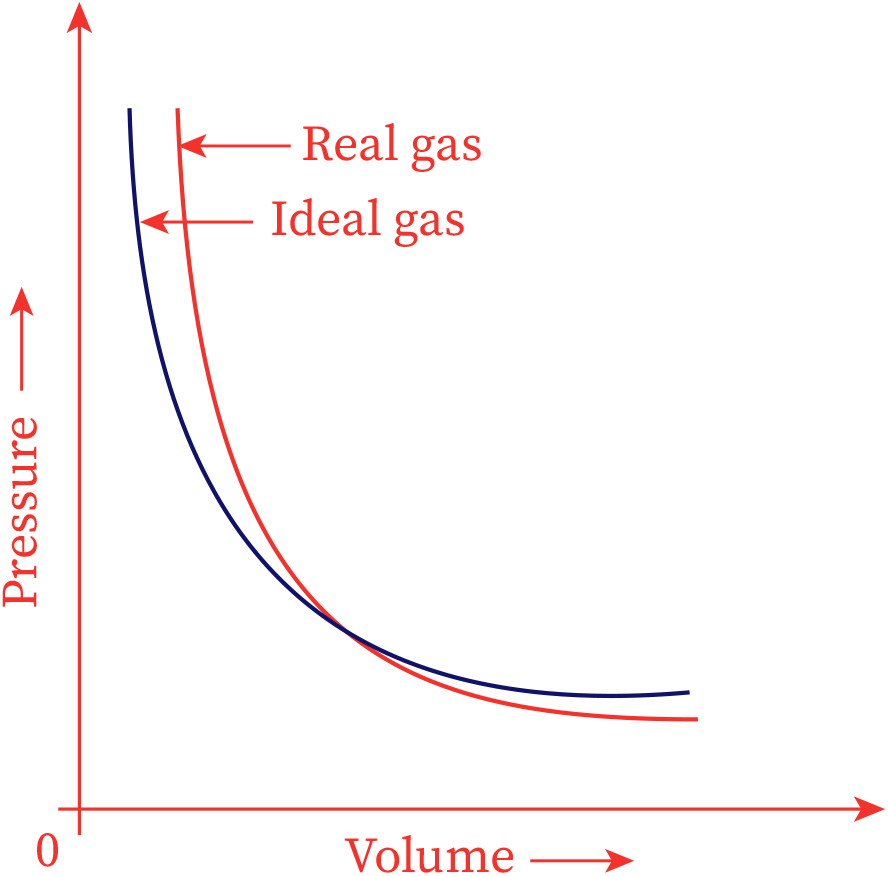
Are Ideal Gases Real . Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction. If you have come straight to this.
from www.bartleby.com
although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. If you have come straight to this. no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions.
Ideal and Real Gases bartleby
Are Ideal Gases Real a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. If you have come straight to this. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it.
From www.studypool.com
SOLUTION Real and ideal gases Studypool Are Ideal Gases Real no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. Fortunately, at the conditions of temperature. Are Ideal Gases Real.
From www.studocu.com
Phy215wk2 real and ideal gases difinition Ideal Gases Experimental Are Ideal Gases Real although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. Fortunately, at the conditions of temperature. Are Ideal Gases Real.
From www.studypool.com
SOLUTION Real and ideal gases Studypool Are Ideal Gases Real Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and. Are Ideal Gases Real.
From classnotes.org.in
Real Gases Chemistry, Class 11, States of Matter Are Ideal Gases Real If you have come straight to this. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures,. Are Ideal Gases Real.
From scienceinfo.com
Ideal Gas Equation Ideal Gas and Laws Are Ideal Gases Real no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction.. Are Ideal Gases Real.
From depositphotos.com
Ideal Gas Intermolecular Forces Real Gas Attractive Forces Stock Vector Are Ideal Gases Real Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. If you have come straight to this. a real gas is defined. Are Ideal Gases Real.
From www.slideserve.com
PPT Properties of Gases PowerPoint Presentation, free download ID Are Ideal Gases Real Gases most closely approximate ideal gas behavior at high temperatures and low pressures. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation.. Are Ideal Gases Real.
From www.bartleby.com
Ideal and Real Gases bartleby Are Ideal Gases Real If you have come straight to this. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. this page looks at how and why real gases differ from ideal gases, and takes a brief. Are Ideal Gases Real.
From www.slideserve.com
PPT REAL VS IDEAL GASES PowerPoint Presentation, free download ID Are Ideal Gases Real Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. Gases most closely approximate ideal gas behavior at high temperatures. Are Ideal Gases Real.
From www.slideserve.com
PPT Ch. 13 Notes Gas Laws PowerPoint Presentation, free download Are Ideal Gases Real no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal. Are Ideal Gases Real.
From www.youtube.com
Difference between Ideal and Real Gas YouTube Are Ideal Gases Real although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction. If you have come straight to this. Fortunately, at the conditions of temperature and pressure that are normally encountered. Are Ideal Gases Real.
From chemistry.stackexchange.com
physical chemistry Pressure vs volume plot for real gas and ideal gas Are Ideal Gases Real this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. If you have come straight to this. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. a real gas is defined. Are Ideal Gases Real.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID5702884 Are Ideal Gases Real Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. Gases most closely approximate ideal gas behavior at high temperatures and low pressures.. Are Ideal Gases Real.
From www.expii.com
Real vs. Ideal Gases — Comparison & Importance Expii Are Ideal Gases Real although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction. no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions.. Are Ideal Gases Real.
From www.slideserve.com
PPT GASES PowerPoint Presentation, free download ID4186388 Are Ideal Gases Real this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. although no gas has these properties, the behaviour of real gases is. Are Ideal Gases Real.
From www.youtube.com
Ideal Gas Vs Real Gas YouTube Are Ideal Gases Real Gases most closely approximate ideal gas behavior at high temperatures and low pressures. If you have come straight to this. no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law. Are Ideal Gases Real.
From www.youtube.com
Difference Between Real Gases and Ideal Gases YouTube Are Ideal Gases Real If you have come straight to this. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. although no gas has these. Are Ideal Gases Real.
From sciencenotes.org
Ideal Gas Law Formula and Examples Are Ideal Gases Real this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. If you have come straight to this. a real gas is defined as a gas that does not obey gas laws. Are Ideal Gases Real.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 Are Ideal Gases Real Gases most closely approximate ideal gas behavior at high temperatures and low pressures. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases.. Are Ideal Gases Real.
From www.slideserve.com
PPT Chapter 14 Properties of Gases PowerPoint Presentation, free Are Ideal Gases Real Gases most closely approximate ideal gas behavior at high temperatures and low pressures. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures. Are Ideal Gases Real.
From www.britannica.com
Equation of state Definition, Ideal Gas, & Facts Britannica Are Ideal Gases Real If you have come straight to this. no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules. Are Ideal Gases Real.
From www.slideserve.com
PPT REAL VS IDEAL GASES PowerPoint Presentation, free download ID Are Ideal Gases Real we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. this page looks at how and why real gases differ from ideal gases, and takes a brief look. Are Ideal Gases Real.
From www.youtube.com
Ideal vs Real Gases YouTube Are Ideal Gases Real we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. although no gas has these properties, the behaviour of real gases is described quite closely. Are Ideal Gases Real.
From energiatoday.com
Ideal Gas vs Real Gas Differences and Concept Are Ideal Gases Real we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases.. Are Ideal Gases Real.
From www.youtube.com
Condition when real gases behaves as ideal gas YouTube Are Ideal Gases Real no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. If you have come straight to. Are Ideal Gases Real.
From chem.libretexts.org
10.9 Real Gases Deviations from Ideal Behavior Chemistry LibreTexts Are Ideal Gases Real If you have come straight to this. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. although no gas has these. Are Ideal Gases Real.
From chem.libretexts.org
Chapter 11.1 Real Gases Chemistry LibreTexts Are Ideal Gases Real If you have come straight to this. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. although no gas has these properties, the behaviour of real gases is described quite. Are Ideal Gases Real.
From www.shutterstock.com
Ideal Gas Real Gas Diagram Scientific Stock Vector (Royalty Free Are Ideal Gases Real Gases most closely approximate ideal gas behavior at high temperatures and low pressures. Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. If you have come straight to this. a real gas is defined as a gas that does not obey gas laws. Are Ideal Gases Real.
From www.slideserve.com
PPT Chapter 11 Gases PowerPoint Presentation, free download ID6309254 Are Ideal Gases Real Gases most closely approximate ideal gas behavior at high temperatures and low pressures. If you have come straight to this. no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law. Are Ideal Gases Real.
From byjus.com
What is ideal gas and real gas? Are Ideal Gases Real If you have come straight to this. we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. this page looks at how and why real gases differ from ideal gases, and takes a brief. Are Ideal Gases Real.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID Are Ideal Gases Real although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction. a real gas is defined as a gas that does not obey gas laws at all standard pressure. Are Ideal Gases Real.
From www.slideserve.com
PPT Unit 6 Gases & The Molecular Theory PowerPoint Are Ideal Gases Real we can reasonably say that we can best understand the behavior of a real gas by understanding how and why it. If you have come straight to this. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. a real gas is defined as a gas that does not obey gas laws at all standard. Are Ideal Gases Real.
From sciencenotes.org
Real Gas vs Ideal Gas Are Ideal Gases Real no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. If you have come straight to this. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. Gases most closely approximate ideal gas behavior at high. Are Ideal Gases Real.
From www.youtube.com
The Ideal Gas Law YouTube Are Ideal Gases Real If you have come straight to this. Gases most closely approximate ideal gas behavior at high temperatures and low pressures. a real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. we can reasonably say that we can best understand the behavior of a real gas by understanding. Are Ideal Gases Real.
From www.slideserve.com
PPT Chapter 13 Gases PowerPoint Presentation, free download ID6371960 Are Ideal Gases Real no real gas exhibits ideal gas behavior, although many real gases approximate it over a range of conditions. this page looks at how and why real gases differ from ideal gases, and takes a brief look at the van der waals equation. If you have come straight to this. Fortunately, at the conditions of temperature and pressure that. Are Ideal Gases Real.