Ce Marking Medical Devices Europe . One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. Your guide to european ce marks for medical devices. The ce marking should be affixed to the device or its sterile packaging. Launching a new medical device is a highly regulated process whether you are. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). The ce marking indicates that the legal. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. These products fall under the medical.
from ce-marking.com
The ce marking indicates that the legal. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. These products fall under the medical. The ce marking should be affixed to the device or its sterile packaging. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Launching a new medical device is a highly regulated process whether you are. Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant.
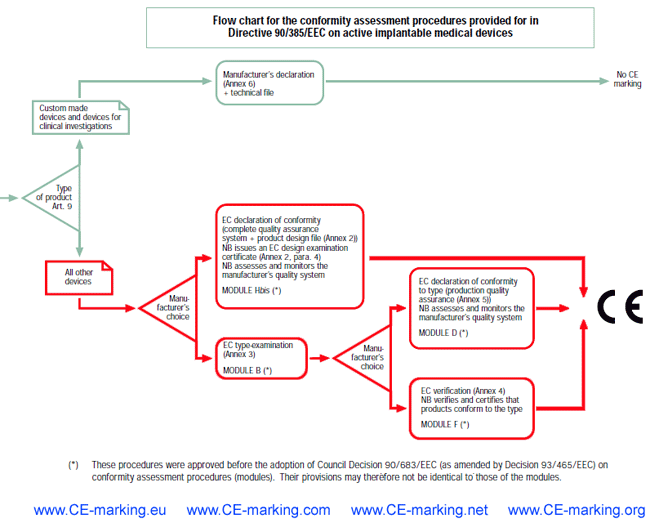
Guide on AIMDActive Implantable Medical Devices CE marking (mark) & European (EU) Authorized
Ce Marking Medical Devices Europe One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. The ce marking should be affixed to the device or its sterile packaging. The ce marking indicates that the legal. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. Your guide to european ce marks for medical devices. Launching a new medical device is a highly regulated process whether you are. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. These products fall under the medical. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations.
From www.pngwing.com
European Union CE marking Directive Medical device European Commission, others, text, trademark Ce Marking Medical Devices Europe These products fall under the medical. Launching a new medical device is a highly regulated process whether you are. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). Regarding medical devices, the ce marking allows companies to move and sell their devices across. Ce Marking Medical Devices Europe.
From www.compliancequest.com
A Guide to CE Marking for Medical Devices Ce Marking Medical Devices Europe One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device. Ce Marking Medical Devices Europe.
From operonstrategist.com
Guide to Obtaining CE Marking for Medical Devices (Navigate EU Regulations Successfully Ce Marking Medical Devices Europe Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Launching a new medical device is a highly regulated process whether you are. The ce. Ce Marking Medical Devices Europe.
From www.youtube.com
7 Steps How to Get a CE Marking Certification for Medical Devices? YouTube Ce Marking Medical Devices Europe Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). The ce marking should be affixed to the device or its sterile packaging. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european. Ce Marking Medical Devices Europe.
From operonstrategist.com
CE Mark for Medical Devices CE Certification (Requirement & Approval) Guidance Operon Strategist Ce Marking Medical Devices Europe The ce marking indicates that the legal. Launching a new medical device is a highly regulated process whether you are. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. One of the essential requirements for selling medical devices in the. Ce Marking Medical Devices Europe.
From www.techsollifesciences.com
Product Certification CE Marking Techsol Life Sciences Ce Marking Medical Devices Europe One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. The ce marking indicates that the legal. A medical device may contain an ancillary. Ce Marking Medical Devices Europe.
From www.pinterest.com
Pin op CE Marking Ce Marking Medical Devices Europe The ce marking indicates that the legal. Launching a new medical device is a highly regulated process whether you are. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. One of the essential. Ce Marking Medical Devices Europe.
From www.rimsys.io
CE marking guide for medical devices in the European Union Ce Marking Medical Devices Europe Your guide to european ce marks for medical devices. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Ce marking is the medical device manufacturer’s claim that a product meets the general safety. Ce Marking Medical Devices Europe.
From www.greenlight.guru
CE Marking for Medical Devices with EU MDR Requirements (5 Steps) Ce Marking Medical Devices Europe These products fall under the medical. The ce marking indicates that the legal. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Launching a. Ce Marking Medical Devices Europe.
From www.ce-marking.com
CE mark medical device medical devices CE marking Ce Marking Medical Devices Europe The ce marking should be affixed to the device or its sterile packaging. These products fall under the medical. Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. A medical. Ce Marking Medical Devices Europe.
From blog.sierralabs.com
5 Steps to Obtain a CE Marking on Your Medical Device Ce Marking Medical Devices Europe Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european. Ce Marking Medical Devices Europe.
From www.rimsys.io
CE marking guide for medical devices in the EU Ce Marking Medical Devices Europe The ce marking indicates that the legal. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. The ce marking should be affixed to the device or its sterile packaging. One of the essential requirements for selling medical devices in the. Ce Marking Medical Devices Europe.
From www.polarseal.net
Polarseal CE Marking Medical Devices Medical Device Manufacturer Ce Marking Medical Devices Europe The ce marking should be affixed to the device or its sterile packaging. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Launching a new medical device is a highly regulated process whether you are. A medical device may contain an ancillary medicinal. Ce Marking Medical Devices Europe.
From operonstrategist.com
CE Mark Consultant for Medical Device (CE Certification & Approvals) in India Operon Strategist Ce Marking Medical Devices Europe One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance. Ce Marking Medical Devices Europe.
From www.ghanayello.com
CE Mark for Medical Device CE Mark (CPR) Construction Products Regulation Ce Marking Medical Devices Europe Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Launching a new medical device is a highly regulated process whether you are. The ce marking should. Ce Marking Medical Devices Europe.
From www.desertcart.in
Buy Medical Devices EU Directives, Guidance Documents, CE Marking Process, ISO Certification Ce Marking Medical Devices Europe Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). These products fall under the. Ce Marking Medical Devices Europe.
From essenvia.com
Your Guide to European CE Mark for Medical Devices Essenvia Ce Marking Medical Devices Europe A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). The ce marking should be affixed to the device or its sterile packaging. Medical devices placed in. Ce Marking Medical Devices Europe.
From www.slideshare.net
Europe CE Marking for medical devices under new MDR Ce Marking Medical Devices Europe A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device. Ce Marking Medical Devices Europe.
From www.rimsys.io
CE marking guide for medical devices in the European Union Ce Marking Medical Devices Europe Launching a new medical device is a highly regulated process whether you are. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. The ce marking indicates. Ce Marking Medical Devices Europe.
From ce-marking.net
Guide on Class IIb MDD Medical Devices CE marking (mark) & European (EU) Authorized Ce Marking Medical Devices Europe Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). The ce marking indicates that the legal. Regarding medical devices, the ce marking allows companies. Ce Marking Medical Devices Europe.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark) & European (EU) Authorized Ce Marking Medical Devices Europe Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. Your guide to european ce marks for medical devices. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Commercializing medical devices in the european union (eu) requires a ce marking. Ce Marking Medical Devices Europe.
From kc-prof.ru
CE certification of medical devices Ce Marking Medical Devices Europe Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Launching a new medical device is a highly regulated process whether you. Ce Marking Medical Devices Europe.
From medicaldevicehq.com
CEmark a medical device How do you do it? Ce Marking Medical Devices Europe Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. Your guide to european ce marks for medical devices. One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. A medical device may contain. Ce Marking Medical Devices Europe.
From www.freyrsolutions.com
Medical Devices and CE Marking Process under the EU MDR Freyr Global Regulatory Solutions Ce Marking Medical Devices Europe Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. Launching a new medical device is a highly regulated. Ce Marking Medical Devices Europe.
From www.i3cglobal.uk
CE Mark Medical Devices Consultants in UK Ce Marking Medical Devices Europe Your guide to european ce marks for medical devices. Launching a new medical device is a highly regulated process whether you are. The ce marking indicates that the legal. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Regarding medical devices, the ce marking allows companies to move and sell. Ce Marking Medical Devices Europe.
From ce-marking.com
Guide on AIMDActive Implantable Medical Devices CE marking (mark) & European (EU) Authorized Ce Marking Medical Devices Europe Launching a new medical device is a highly regulated process whether you are. Your guide to european ce marks for medical devices. Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. A medical device may contain an ancillary medicinal substance. Ce Marking Medical Devices Europe.
From www.youtube.com
CE Marking Process as per EU MDR (European Medical Device Regulation) YouTube Ce Marking Medical Devices Europe Launching a new medical device is a highly regulated process whether you are. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Commercializing medical devices in the european union (eu) requires a ce marking demonstrating compliance with the medical device regulations. Medical devices placed in the eu market must be labelled with. Ce Marking Medical Devices Europe.
From operonstrategist.com
EUMDR Affected CE Marking Complete Guide for Medical Device Manufacturers Operon Strategist Ce Marking Medical Devices Europe Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. Ce Marking Medical Devices Europe.
From www.emergobyul.com
Europe CE Marking Regulatory Process for Medical Devices Emergo by UL Ce Marking Medical Devices Europe Your guide to european ce marks for medical devices. Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. The ce marking should be affixed to the. Ce Marking Medical Devices Europe.
From operonstrategist.com
European CE Mark for Medical Devices CE Certification Guidance (Quick Process of Approval Ce Marking Medical Devices Europe Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Regarding medical devices, the ce marking allows companies to move and sell their devices across the 30 countries of the european economic area (eea) once they follow the eu. A medical device may contain. Ce Marking Medical Devices Europe.
From www.ce-marking.com
Guide on Class III MDD Medical Devices CE marking (mark) & European (EU) Authorized Ce Marking Medical Devices Europe The ce marking should be affixed to the device or its sterile packaging. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. One of the essential requirements for selling medical devices in the eu is obtaining the ce marking. Medical devices placed in the eu market must be labelled with the ce. Ce Marking Medical Devices Europe.
From operonstrategist.com
Medical Device CE Mark Consultant in Egypt (CE Mark Certification and Approvals) Operon Strategist Ce Marking Medical Devices Europe Launching a new medical device is a highly regulated process whether you are. These products fall under the medical. The ce marking should be affixed to the device or its sterile packaging. A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Ce marking is the medical device manufacturer’s claim that a product. Ce Marking Medical Devices Europe.
From us.operonstrategist.com
CE Mark for Medical Devices (StepbyStep Process) Operon Strategist Ce Marking Medical Devices Europe A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). The ce marking indicates that the legal. One of the essential requirements for selling medical devices in. Ce Marking Medical Devices Europe.
From www.kolabtree.com
医療機器のCEマークを取得するには(EU MDR Ce Marking Medical Devices Europe Medical devices manufacturers willing to market products in the european union (eu), must comply with the regulations established by the european union medical device regulation (mdr). Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. Launching a new medical device is a highly regulated process whether you. Ce Marking Medical Devices Europe.
From operonstrategist.com
European CE Marking for Medical Devices CE Certification Guidance (Process of Approval Ce Marking Medical Devices Europe Medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of the medical device regulation (mdr). Ce marking is the medical device manufacturer’s claim that a product meets the general safety and performance requirements (gspr) of all relevant. The ce marking indicates that the legal. One of the essential requirements. Ce Marking Medical Devices Europe.