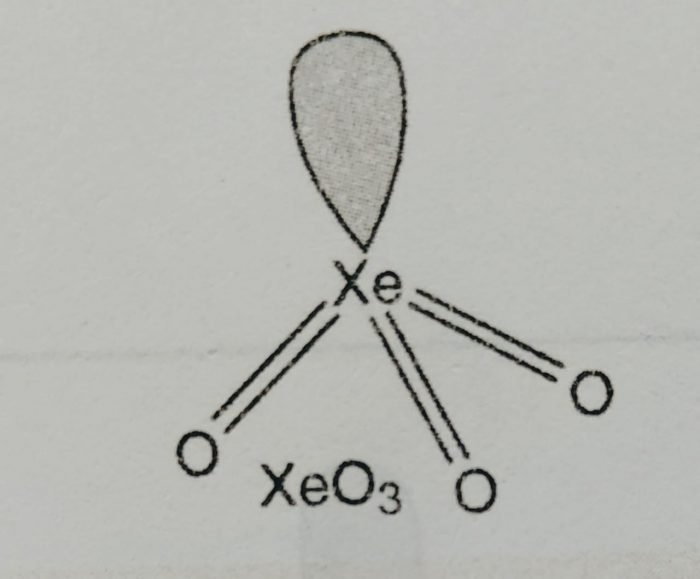
Why Xenon Form Compound . In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. It reacts with highly electronegative and small sized fluorine (and oxygen).
from classnotes.org.in
Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Hence, the valence electron of xe is. It reacts with highly electronegative and small sized fluorine (and oxygen). In large sized xenon, the electron attraction to the nucleus is weaker. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an.
Compounds of Xenon and uses of Noble Gases Chemistry, Class 12, The p
Why Xenon Form Compound Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. It reacts with highly electronegative and small sized fluorine (and oxygen). Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride.
From www.researchgate.net
Simplified energy diagram of xenon [39]. Download Scientific Diagram Why Xenon Form Compound It reacts with highly electronegative and small sized fluorine (and oxygen). Hence, the valence electron of xe is. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as. Why Xenon Form Compound.
From www.examples.com
What is Xenon(Xe)? Preparation, Properties, Uses, Compounds, Reactivity Why Xenon Form Compound According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Hence, the valence electron of xe is. It reacts with highly electronegative and small sized fluorine (and oxygen). Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as. Why Xenon Form Compound.
From www.pinterest.com
Xenon Compounds Chemistry, Noble gas, Science and nature Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Hence, the valence electron of xe is. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. In large sized xenon, the electron attraction to the. Why Xenon Form Compound.
From www.youtube.com
Structures of compounds of Xenon YouTube Why Xenon Form Compound It reacts with highly electronegative and small sized fluorine (and oxygen). In large sized xenon, the electron attraction to the nucleus is weaker. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and. Why Xenon Form Compound.
From classnotes.org.in
Compounds of Xenon and uses of Noble Gases Chemistry, Class 12, The p Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. It reacts with highly electronegative and small sized fluorine (and oxygen). Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases. Why Xenon Form Compound.
From www.toppr.com
Xenon hexafluoride on partial hydrolysis produces compounds 'X' and 'Y Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Hence, the valence electron of xe is. Specifically, he predicted the existence of krypton hexafluoride (kr. Why Xenon Form Compound.
From www.youtube.com
Xenon compoundsStructure YouTube Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. It reacts with highly electronegative and small sized fluorine (and oxygen). Specifically, he predicted the existence. Why Xenon Form Compound.
From byjus.com
What type of bond does Xenon forms? Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as. Why Xenon Form Compound.
From wireliststatolith.z13.web.core.windows.net
A Diagram Of Xenon Why Xenon Form Compound According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Hence, the valence electron of xe is. It reacts with highly electronegative and small sized fluorine. Why Xenon Form Compound.
From fyoozmdck.blob.core.windows.net
Xenon Krypton Compound at Irene Hathaway blog Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds. Why Xenon Form Compound.
From www.examples.com
Xenon (Xe) Definition, Preparation, Properties, Uses, Compounds Why Xenon Form Compound According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist. Why Xenon Form Compound.
From www.scribd.com
Compounds of Xenon PDF PDF Why Xenon Form Compound Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. In large sized xenon, the electron attraction to the nucleus is weaker. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. It reacts with highly electronegative. Why Xenon Form Compound.
From www.youtube.com
xenon compounds by tushar sr. no.41 roll no. 1221271090050 YouTube Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. It reacts with highly electronegative and small sized fluorine (and oxygen). Learn how neil bartlett, a canadian chemist, disproved the inertness of. Why Xenon Form Compound.
From www.numerade.com
Why does xenon form stable compounds with fluorine, whereas argon does Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Hence, the valence electron of xe is. In large sized xenon, the electron attraction to the. Why Xenon Form Compound.
From www.youtube.com
Xenon compounds preparation, properties, structures YouTube Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. According to the octet rule noble gases should not form compounds. Why Xenon Form Compound.
From askfilo.com
Table 7.13 Structure of Xenon Compounds ∞⋄∞161⊗∞∞⊗ Filo Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. It reacts with highly electronegative and small sized fluorine (and oxygen). Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. Hence, the valence electron of xe is. Learn how neil bartlett, a canadian. Why Xenon Form Compound.
From www.doubtnut.com
[Odia] Why does xenon form compounds only with fluorine and oxygen Why Xenon Form Compound Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. Hence, the valence electron of xe is. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. According to the octet. Why Xenon Form Compound.
From www.vedantu.com
Draw the structure of xenon hexafluoride (Xe{{F}_{6}}) molecule and Why Xenon Form Compound Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of. Why Xenon Form Compound.
From studiousguy.com
Xenon (Xe) Properties & Uses StudiousGuy Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. It reacts with highly electronegative and small sized fluorine (and oxygen). Specifically, he predicted the existence of krypton hexafluoride (kr f 6). Why Xenon Form Compound.
From www.examples.com
Xenon (Xe) Definition, Preparation, Properties, Uses, Compounds Why Xenon Form Compound Hence, the valence electron of xe is. It reacts with highly electronegative and small sized fluorine (and oxygen). In large sized xenon, the electron attraction to the nucleus is weaker. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Specifically, he predicted the existence. Why Xenon Form Compound.
From quizlet.com
Xenon forms wellcharacterized compounds (4 page 400). Two x Quizlet Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. In large sized xenon, the electron attraction to the nucleus is weaker. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Specifically, he predicted the. Why Xenon Form Compound.
From alchetron.com
Xenon tetrafluoride Alchetron, The Free Social Encyclopedia Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist. Why Xenon Form Compound.
From www.slideshare.net
Chemistry presentation on xenon compounds Why Xenon Form Compound Hence, the valence electron of xe is. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. It reacts with highly electronegative and small sized fluorine (and oxygen). According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like. Why Xenon Form Compound.
From www.thoughtco.com
Noble Gas Chemical Compounds Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. Hence, the valence electron of. Why Xenon Form Compound.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID6301153 Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Hence, the valence electron of xe is. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Specifically, he predicted the existence of krypton hexafluoride (kr. Why Xenon Form Compound.
From www.slideserve.com
PPT The Noble Gases PowerPoint Presentation, free download ID6532925 Why Xenon Form Compound Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. It reacts with highly electronegative and small sized fluorine (and oxygen).. Why Xenon Form Compound.
From studiousguy.com
Xenon (Xe) Properties & Uses StudiousGuy Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. It reacts with highly electronegative and small sized fluorine (and oxygen). Hence, the valence electron of xe is. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated. Why Xenon Form Compound.
From www.sciencephoto.com
Xenon, atomic structure Stock Image C023/2560 Science Photo Library Why Xenon Form Compound It reacts with highly electronegative and small sized fluorine (and oxygen). Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Hence, the valence electron of xe is. In large sized xenon, the electron attraction to the nucleus is weaker. Specifically, he predicted the existence. Why Xenon Form Compound.
From classnotes.org.in
Compounds of Xenon and uses of Noble Gases Chemistry, Class 12, The p Why Xenon Form Compound Hence, the valence electron of xe is. It reacts with highly electronegative and small sized fluorine (and oxygen). Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. In large sized xenon, the electron attraction to the nucleus is weaker. Learn how neil bartlett, a canadian. Why Xenon Form Compound.
From www.toppr.com
Xenon form compounds with fluorine under different conditions. The Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds like $\ce{xef2}$,. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. Learn how neil bartlett, a. Why Xenon Form Compound.
From www.britannica.com
Xenon Definition, Properties, Atomic Mass, Compounds, & Facts Why Xenon Form Compound Hence, the valence electron of xe is. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. In large sized xenon, the electron attraction to the nucleus is weaker. It reacts with highly electronegative and small sized fluorine (and oxygen). Learn how neil bartlett, a canadian. Why Xenon Form Compound.
From www.youtube.com
Geometry of XeO4, XeOF2(Xenon compound)VSEPR TheoryChemical bonding Why Xenon Form Compound Hence, the valence electron of xe is. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. In large sized xenon, the electron attraction to the nucleus is weaker. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds. Why Xenon Form Compound.
From www.slideserve.com
PPT The Trip of One Xenon Atom Through an Ion Engine PowerPoint Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. Hence, the valence electron of xe is. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. It reacts with highly. Why Xenon Form Compound.
From www.chegg.com
Solved Inert xenon actually forms many compounds, especially Why Xenon Form Compound Learn how neil bartlett, a canadian chemist, disproved the inertness of noble gases by making the first noble gas compound with xenon and platinum hexafluoride. In large sized xenon, the electron attraction to the nucleus is weaker. Hence, the valence electron of xe is. According to the octet rule noble gases should not form compounds but xenon and krypton form. Why Xenon Form Compound.
From byjus.com
A xenon compound ‘A’ upon partial hydrolysis gives XeO2F2. The number Why Xenon Form Compound In large sized xenon, the electron attraction to the nucleus is weaker. Specifically, he predicted the existence of krypton hexafluoride (kr f 6) and xenon hexafluoride (xef 6), speculated that xef 8 might exist as an. Hence, the valence electron of xe is. According to the octet rule noble gases should not form compounds but xenon and krypton form compounds. Why Xenon Form Compound.