Organic Solvent Gas Solubility . It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. of gases in liquids is arbitrary. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c.
from www.dreamstime.com
beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such.
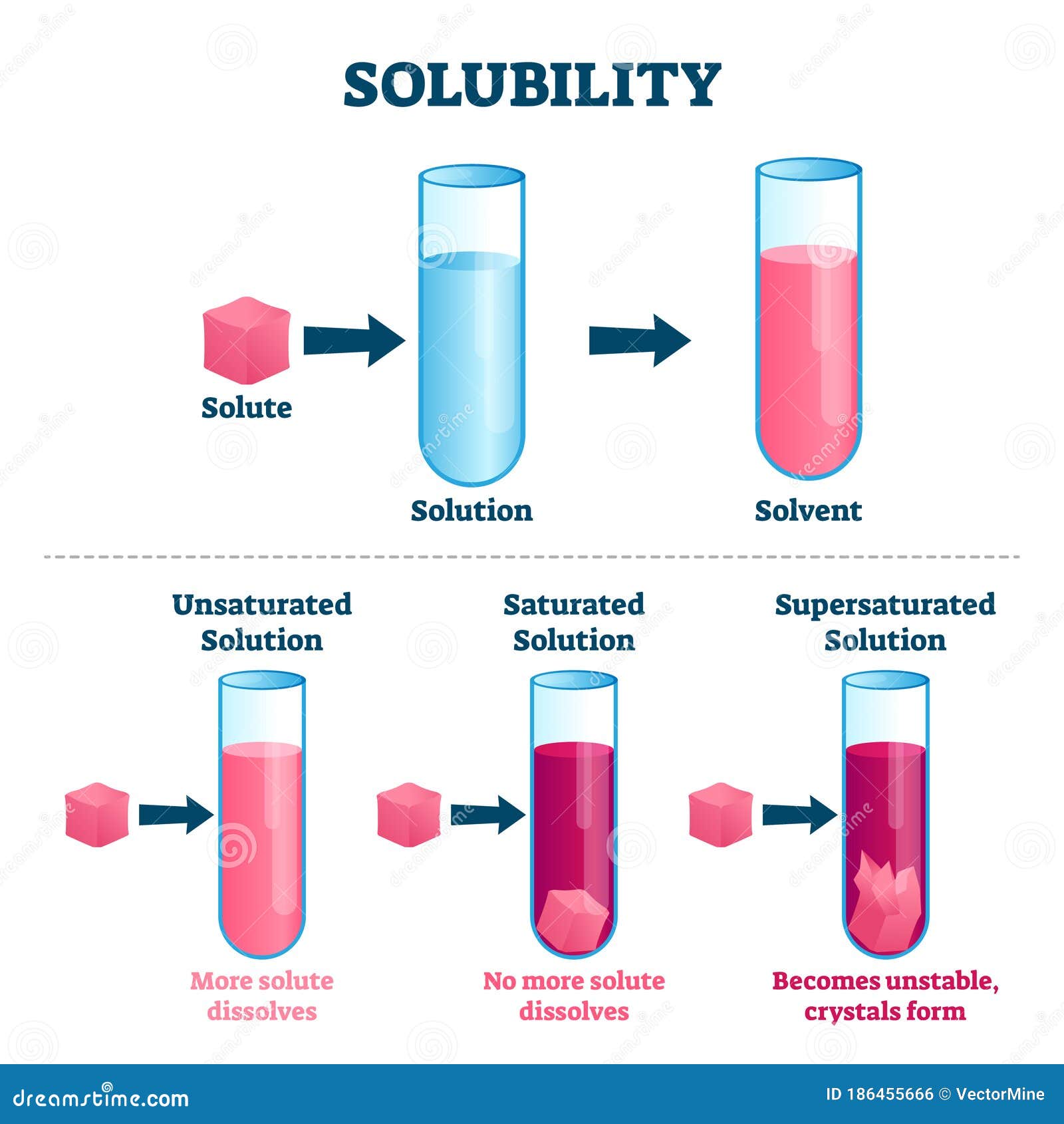
Solubility Vector Illustration. Labeled Solute, Solvent and Solution
Organic Solvent Gas Solubility beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. of gases in liquids is arbitrary. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Organic Solvent Gas Solubility beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k. Organic Solvent Gas Solubility.
From www.researchgate.net
Shows the list of common organic solvents, their formula and melting Organic Solvent Gas Solubility \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. of gases in liquids is arbitrary. the solubility of oxygen in 21 pure. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Organic Solvent Gas Solubility \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. . Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Organic Solvent Gas Solubility considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33. Organic Solvent Gas Solubility.
From pubs.acs.org
Solubility of Electrolytes in Organic Solvents SolventSpecific Organic Solvent Gas Solubility beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. It is generally accepted that the equilibrium set up at 300k between a typical gas. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solubility and Extraction PowerPoint Presentation, free download Organic Solvent Gas Solubility considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. of gases in liquids is arbitrary. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and. Organic Solvent Gas Solubility.
From www.researchgate.net
Solubility and gelation properties of BHPBIA in various organic Organic Solvent Gas Solubility It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. of gases in liquids is arbitrary. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. \[ \delta + \ce{ solute (gas) + solvent (solvent). Organic Solvent Gas Solubility.
From pubs.acs.org
Solubility of Electrolytes in Organic Solvents SolventSpecific Organic Solvent Gas Solubility the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. of gases in liquids is arbitrary. \[ \delta + \ce{ solute (gas) + solvent. Organic Solvent Gas Solubility.
From www.researchgate.net
Selected Data on Ozone Solubility in Organic Solvents Download Table Organic Solvent Gas Solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. solubilities of the highly soluble gases, propane. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Organic Solvent Gas Solubility solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. of gases in liquids is arbitrary. beginning with volume 1 covering the solubility of helium and neon in 1979 work. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID6260427 Organic Solvent Gas Solubility It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. of gases in liquids is arbitrary. considering the role of the solvent’s chemical structure,. Organic Solvent Gas Solubility.
From www.semanticscholar.org
Table 1 from Solubility of hidrogen and carbon monoxide in water and Organic Solvent Gas Solubility beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. of gases in liquids is arbitrary. considering the role of the solvent’s chemical structure, note that the solubility of. Organic Solvent Gas Solubility.
From sample-templates123.com
How To Read And Understand A Solubility Chart Example Free Sample Organic Solvent Gas Solubility beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents.. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT GAS SOLUBILITY OF HFCs IN ORGANIC SOLVENTS PowerPoint Organic Solvent Gas Solubility It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)}. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Organic Solvent Gas Solubility \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. of gases in liquids is arbitrary. the solubility of oxygen in 21 pure organic. Organic Solvent Gas Solubility.
From mungfali.com
Gas Solubility Curve Organic Solvent Gas Solubility solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c.. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Organic Solvent Gas Solubility solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method.. Organic Solvent Gas Solubility.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Organic Solvent Gas Solubility solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. . Organic Solvent Gas Solubility.
From courses.lumenlearning.com
Gas Solubility and Temperature Introduction to Chemistry Organic Solvent Gas Solubility It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents.. Organic Solvent Gas Solubility.
From ar.inspiredpencil.com
Solvent Solubility Chart Organic Solvent Gas Solubility It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. of gases in liquids is arbitrary. considering the role of the solvent’s chemical. Organic Solvent Gas Solubility.
From www.researchgate.net
O3 Solubility in Various Organic Solvents Download Scientific Diagram Organic Solvent Gas Solubility the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in. Organic Solvent Gas Solubility.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Organic Solvent Gas Solubility \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. of gases in liquids is arbitrary. solubilities of the highly soluble gases, propane. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Organic Solvent Gas Solubility beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Organic Solvent Gas Solubility solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. of gases in liquids is arbitrary. the solubility of oxygen in 21 pure organic solvents was measured at 298.2. Organic Solvent Gas Solubility.
From es.scribd.com
Organic Solvents Data With Water Solubility PDF Solvent Organic Solvent Gas Solubility considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of.. Organic Solvent Gas Solubility.
From courses.lumenlearning.com
Solubility Chemistry for Majors Organic Solvent Gas Solubility the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. of gases in liquids is arbitrary. \[ \delta + \ce{ solute (gas) +. Organic Solvent Gas Solubility.
From www.researchgate.net
(PDF) Calculation of gas solubility in water and various organic Organic Solvent Gas Solubility It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array. Organic Solvent Gas Solubility.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID6014910 Organic Solvent Gas Solubility \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar. Organic Solvent Gas Solubility.
From surfguppy.com
Solubility Surfguppy Chemistry made easy for visual learners Organic Solvent Gas Solubility \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. considering the role of the solvent’s chemical structure, note that the solubility of oxygen. Organic Solvent Gas Solubility.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Organic Solvent Gas Solubility beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. It is generally accepted that the equilibrium set up at 300k between a typical gas. Organic Solvent Gas Solubility.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Organic Solvent Gas Solubility considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\]. Organic Solvent Gas Solubility.
From www.sliderbase.com
Solution Stoichiometry Presentation Chemistry Organic Solvent Gas Solubility solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. considering the role of the solvent’s chemical structure, note that the solubility of oxygen in the liquid hydrocarbon hexane, c. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. . Organic Solvent Gas Solubility.
From www.caymanchem.com
Solubility Rules Chart.png Organic Solvent Gas Solubility It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. \[ \delta + \ce{ solute (gas) + solvent (solvent) \rightleftharpoons solute (sol) + solvent (sol)} \label{eq5}\] where \(delta\) is thermal energy. solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents.. Organic Solvent Gas Solubility.
From ar.inspiredpencil.com
Solubility Chart Chemistry 11 Organic Solvent Gas Solubility the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k and 101.33 kpa using the static method. beginning with volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of. considering the role of the solvent’s chemical structure, note that the solubility of oxygen. Organic Solvent Gas Solubility.
From www.researchgate.net
Solubility of organic compounds in liquid carbon dioxide [21 Organic Solvent Gas Solubility solubilities of the highly soluble gases, propane and butane, in normal paraffin and polar solvents. It is generally accepted that the equilibrium set up at 300k between a typical gas such as argon and a liquid such. of gases in liquids is arbitrary. the solubility of oxygen in 21 pure organic solvents was measured at 298.2 k. Organic Solvent Gas Solubility.