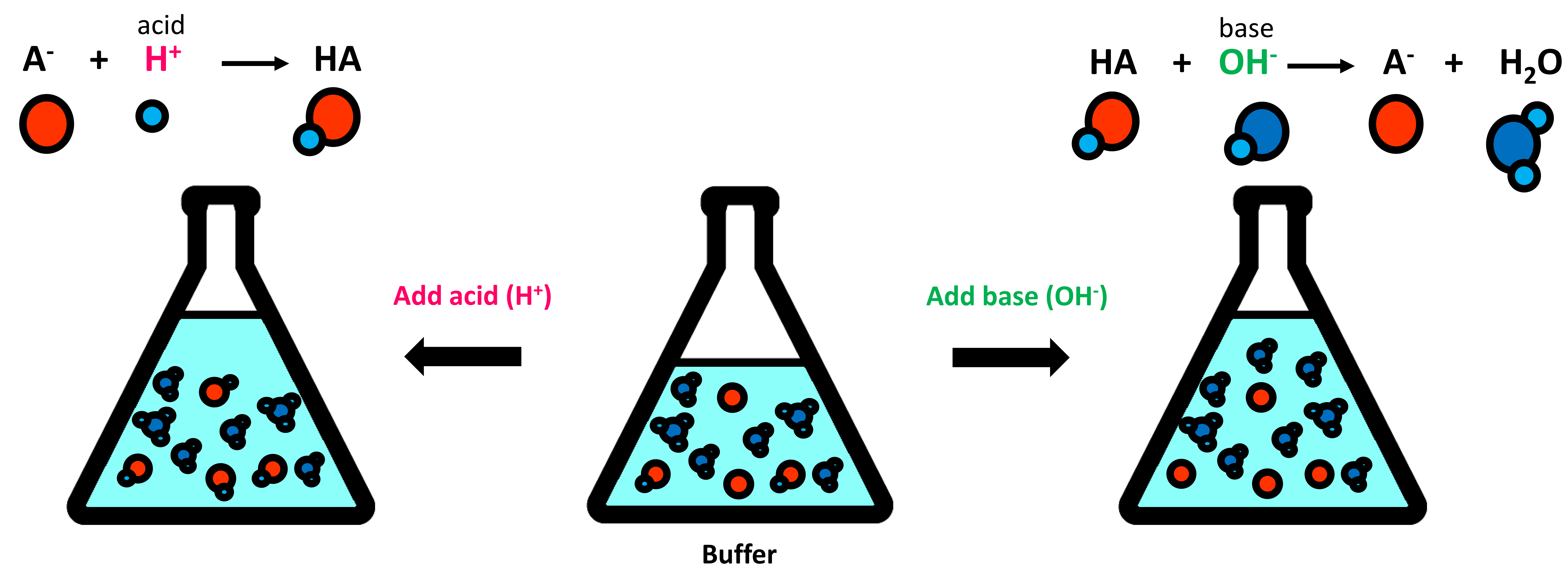
Biological Buffers . A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Four important generalizations about buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.
from deporecipe.co
Four important generalizations about buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems.
10x Protein Running Buffer Recipe Deporecipe.co
Biological Buffers Four important generalizations about buffers. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. Four important generalizations about buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems.
From www.youtube.com
Role of buffers in biological system YouTube Biological Buffers The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. Four important generalizations about buffers. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid. Biological Buffers.
From www.labdepotinc.com
Bicine, Biological Buffer Biological Buffers The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. Learn what biological buffers are, how they help maintain. Biological Buffers.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Biological Buffers The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak. Biological Buffers.
From www.youtube.com
Acid, Base & Buffer in Biological systems YouTube Biological Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and. Biological Buffers.
From www.labdepotinc.com
HEPES, Biological Buffer Biological Buffers In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The purpose of a buffer in a biological system is to maintain intracellular and. Biological Buffers.
From agscientific.com
5 Things to Consider When Choosing Biological Buffers Biological Buffers Four important generalizations about buffers. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Biological Buffers.
From www.fishersci.se
Thermo Scientific™ Pierce™ IgG Elution Buffer Biological Buffers Life Biological Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. Four important generalizations about buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and. Biological Buffers.
From www.protocols.io
Buffers for Use in Biological Systems Biological Buffers The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Biological Buffers.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Biological Buffers The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Four important generalizations about buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system,. Biological Buffers.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Buffers In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Four important generalizations about buffers. A buffer is composed of an equilibrium mixture of a weak. Biological Buffers.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Biological Buffers Four important generalizations about buffers. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. Learn what biological buffers are, how they help maintain. Biological Buffers.
From www.youtube.com
BUFFERS IN PHARMACEUTICAL AND BIOLOGICAL SYSTEM BUFFER pH Biological Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls. Biological Buffers.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffers Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which. Biological Buffers.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Biological Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.. Biological Buffers.
From www.gbiosciences.com
Molecular Biology Buffers & Chemicals GBiosciences Biological Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Four important generalizations about buffers. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical. Biological Buffers.
From www.gbiosciences.com
Molecular Biology Buffers & Chemicals GBiosciences Biological Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range. Biological Buffers.
From teknova.com
Antifoam Teknova Teknova Biological Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. Four important generalizations about buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and. Biological Buffers.
From www.labdepotinc.com
ACES Biological Buffer Biological Buffers In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of an equilibrium mixture of a weak acid (ha) and. Biological Buffers.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Biological Buffers.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Buffers The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. The buffer. Biological Buffers.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Biological Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Four important generalizations about buffers.. Biological Buffers.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Biological Buffers Four important generalizations about buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical. Biological Buffers.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Biological Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. Four important generalizations about buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. Biological Buffers.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Biological Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Four important generalizations about buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Biological Buffers.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every Biological Buffers The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Four important generalizations about buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Learn what biological buffers are, how they help maintain the body's ph,. Biological Buffers.
From www.biobasic.com
Common Buffers Biological Buffers Biochemicals Products Biological Buffers The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is composed. Biological Buffers.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Buffers Four important generalizations about buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of an equilibrium mixture of. Biological Buffers.
From www.emsdiasum.com
Hepes, Crystal, Biological Buffer Biological Buffers The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.. Biological Buffers.
From www.gbiosciences.com
Molecular Biology Buffers & Chemicals GBiosciences Biological Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Four important generalizations about buffers. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph. Biological Buffers.
From www.reagent.co.uk
How Buffers Maintain Biological Balance The Science Blog Biological Buffers Four important generalizations about buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. The most relevant systems for biology are the. Biological Buffers.
From www.bostonbioproducts.com
Biological Buffers and Process Liquids Boston BioProducts Biological Buffers Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in mammalian systems. Four important generalizations about buffers. A buffer is composed of an equilibrium mixture. Biological Buffers.
From www.youtube.com
Biological Buffers in Nature YouTube Biological Buffers A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have. Biological Buffers.
From www.brainkart.com
Buffers Biological Buffers The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. In this article, you will. Biological Buffers.
From www.labcompare.com
A Guide to Biological Buffer Preparation Biological Buffers Learn what biological buffers are, how they help maintain the body's ph, and why they are important for biochemical processes. Four important generalizations about buffers. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Biological Buffers.
From www.researchgate.net
Examples of buffer compounds divided into accepted physiological Biological Buffers The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Learn what biological buffers are, how they help maintain the body's ph, and why they are important. Biological Buffers.