Table Salt Strong Electrolyte . Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. (the biggest two are sodium. Likewise, a strong acid like hcl splits. The word electrolyte generally refers to the largest concentration of charged particles within the human body. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Electrolytes and sea salt vs table salt. Salt is actually a combination of two key electrolytes — sodium and chloride. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Those elements are among the list of substances classified as electrolytes.
from www.slideserve.com
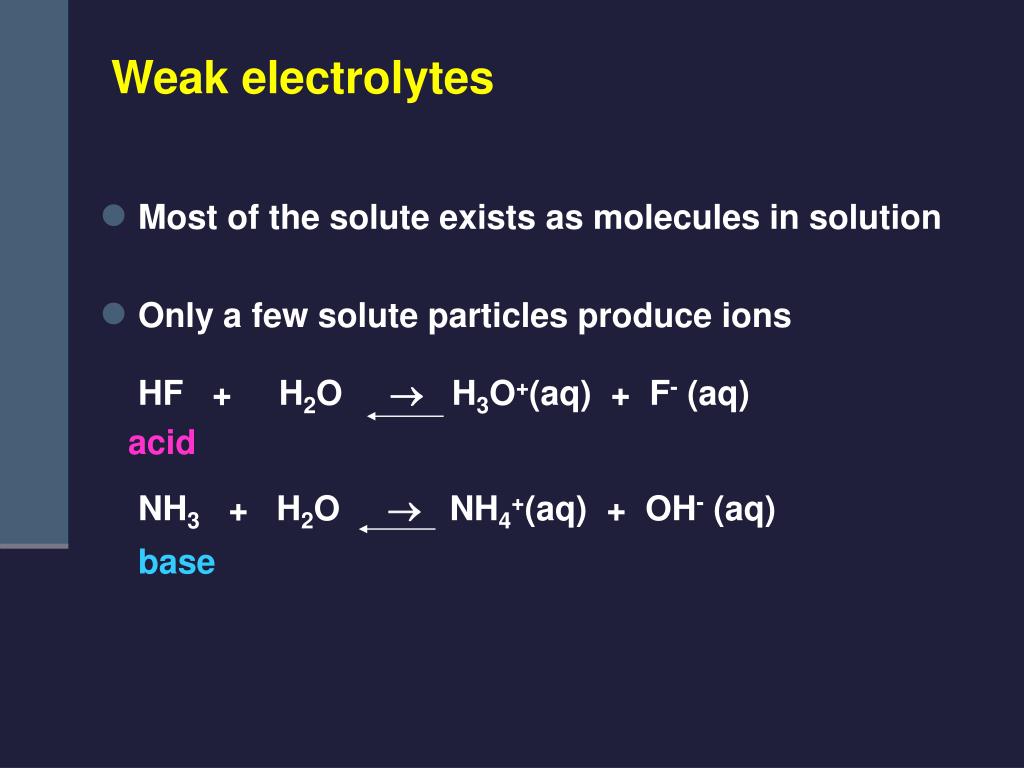
Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. The word electrolyte generally refers to the largest concentration of charged particles within the human body. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. (the biggest two are sodium. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Those elements are among the list of substances classified as electrolytes. Likewise, a strong acid like hcl splits. Salt is actually a combination of two key electrolytes — sodium and chloride. Electrolytes and sea salt vs table salt. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),.
PPT Electrolytes PowerPoint Presentation, free download ID7074703
Table Salt Strong Electrolyte Likewise, a strong acid like hcl splits. Electrolytes and sea salt vs table salt. Likewise, a strong acid like hcl splits. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. (the biggest two are sodium. Those elements are among the list of substances classified as electrolytes. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Salt is actually a combination of two key electrolytes — sodium and chloride. The word electrolyte generally refers to the largest concentration of charged particles within the human body.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL Table Salt Strong Electrolyte A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Those elements are among the list of substances classified as electrolytes. Likewise, a strong acid like hcl splits. Salt is actually a combination of two key electrolytes — sodium and chloride. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Standard issue table salt. Table Salt Strong Electrolyte.
From hxenznaxb.blob.core.windows.net
Can You Use Table Salt For Electrolytes at Debra Parker blog Table Salt Strong Electrolyte Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Electrolytes and sea salt vs table salt. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Salt is actually a combination of two key electrolytes — sodium and chloride.. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Nearly all salts are strong electrolytes. Therefore, salts exist Table Salt Strong Electrolyte Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Those elements are among the list of substances classified as electrolytes. Strong electrolytes completely ionize when dissolved, and no. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Table Salt Strong Electrolyte For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Those elements are among the list of substances classified as electrolytes. Salt is actually a combination of two key electrolytes — sodium and chloride. Electrolytes and sea salt vs table salt. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Likewise, a strong acid like hcl splits. Standard issue table salt contains both sodium. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download Table Salt Strong Electrolyte Those elements are among the list of substances classified as electrolytes. Electrolytes and sea salt vs table salt. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Salt is actually a combination of. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free Table Salt Strong Electrolyte Likewise, a strong acid like hcl splits. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. (the biggest two are sodium. Those elements are among the list of substances classified as electrolytes. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. The word electrolyte generally refers to. Table Salt Strong Electrolyte.
From www.drlaurendeville.com
Electrolytes and Sea Salt vs Table Salt Dr. Lauren Deville Table Salt Strong Electrolyte For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Salt is actually a combination of two key electrolytes — sodium and chloride. Those elements are among the list of substances classified as electrolytes. (the biggest two are sodium. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Likewise, a strong acid like hcl splits. A strong electrolyte, like nacl,. Table Salt Strong Electrolyte.
From ar.inspiredpencil.com
Strong Electrolytes List Table Salt Strong Electrolyte Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Those elements are among the list of substances classified as electrolytes. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Salt is actually a combination of two key electrolytes — sodium and chloride. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. The word electrolyte generally refers to the. Table Salt Strong Electrolyte.
From melissa-media.blogspot.com
Melissa Media Strong And Weak Electrolytes Table Salt Strong Electrolyte Likewise, a strong acid like hcl splits. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of. Table Salt Strong Electrolyte.
From pediaa.com
Difference Between Strong and Weak Electrolytes Definition Table Salt Strong Electrolyte Salt is actually a combination of two key electrolytes — sodium and chloride. Likewise, a strong acid like hcl splits. (the biggest two are sodium. Electrolytes and sea salt vs table salt. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Standard issue table salt. Table Salt Strong Electrolyte.
From www.majordifferences.com
Strong Electrolytes vs Weak Electrolytes Major Differences Table Salt Strong Electrolyte Likewise, a strong acid like hcl splits. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Those elements are among the list of substances. Table Salt Strong Electrolyte.
From www.askdifference.com
Strong Electrolytes vs. Weak Electrolytes — What’s the Difference? Table Salt Strong Electrolyte Salt is actually a combination of two key electrolytes — sodium and chloride. (the biggest two are sodium. Those elements are among the list of substances classified as electrolytes. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. The word electrolyte generally refers. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solutions PowerPoint Presentation Table Salt Strong Electrolyte Those elements are among the list of substances classified as electrolytes. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. (the biggest two are sodium. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Electrolytes and sea salt vs table salt. The word electrolyte generally refers to the largest concentration of charged particles. Table Salt Strong Electrolyte.
From slideserve.com
PPT II. Types of Electrolytes PowerPoint Presentation ID297972 Table Salt Strong Electrolyte Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. The word electrolyte generally refers to the largest concentration of charged particles within the human body. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Those elements are among the list of substances classified as electrolytes. (the biggest two are sodium. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Aqueous Reactions and Solution Stoichiometry PowerPoint Table Salt Strong Electrolyte Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Salt is actually a combination of two key electrolytes — sodium and chloride. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of. Table Salt Strong Electrolyte.
From slideplayer.com
Chapter 4 Aqueous Reactions and Solution Stoichiometry ppt download Table Salt Strong Electrolyte A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Electrolytes and sea salt vs table salt. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. For example,. Table Salt Strong Electrolyte.
From www.nagwa.com
Question Video Identifying Which of Three Substances Is a Strong Table Salt Strong Electrolyte Salt is actually a combination of two key electrolytes — sodium and chloride. (the biggest two are sodium. The word electrolyte generally refers to the largest concentration of charged particles within the human body. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Standard issue table salt contains both sodium and chloride and (consumed in. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Aqueoussolution Reactions PowerPoint Presentation, free download Table Salt Strong Electrolyte Salt is actually a combination of two key electrolytes — sodium and chloride. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Standard issue table salt contains both sodium and chloride and (consumed in moderation). Table Salt Strong Electrolyte.
From www.bouldersaltcompany.com
What Is the Best Salt For POTS? — Boulder Salt Company Table Salt Strong Electrolyte A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Salt is actually a combination of two key electrolytes — sodium and chloride. Those elements are among the list. Table Salt Strong Electrolyte.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy Table Salt Strong Electrolyte (the biggest two are sodium. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Electrolytes and sea salt vs table salt. Electrolyte strength (est). Table Salt Strong Electrolyte.
From general.chemistrysteps.com
Strong and Weak Electrolytes Chemistry Steps Table Salt Strong Electrolyte The word electrolyte generally refers to the largest concentration of charged particles within the human body. Those elements are among the list of substances classified as electrolytes. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Salt is actually a combination of two key electrolytes — sodium and chloride. Electrolyte strength (est) in aqueous solutions,. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Table Salt Strong Electrolyte Salt is actually a combination of two key electrolytes — sodium and chloride. Likewise, a strong acid like hcl splits. Those elements are among the list of substances classified as electrolytes. Electrolytes and sea salt vs table salt. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. (the biggest two are sodium. The word electrolyte generally refers to the largest concentration of charged particles. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I Table Salt Strong Electrolyte Likewise, a strong acid like hcl splits. (the biggest two are sodium. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. The word electrolyte generally refers to the largest concentration of charged particles within the human body. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value. Table Salt Strong Electrolyte.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Table Salt Strong Electrolyte Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. (the biggest two are sodium. Electrolytes and sea salt vs table salt. The word electrolyte generally refers to the largest concentration of charged particles within the human body. Salt is actually a combination of two key. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation ID5407668 Table Salt Strong Electrolyte The word electrolyte generally refers to the largest concentration of charged particles within the human body. Those elements are among the list of substances classified as electrolytes. Likewise, a strong acid like hcl splits. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage.. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Acid, Bases, and Salts PowerPoint Presentation, free download Table Salt Strong Electrolyte Salt is actually a combination of two key electrolytes — sodium and chloride. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Likewise, a strong acid like hcl splits. (the biggest two are sodium. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Strong electrolytes completely ionize. Table Salt Strong Electrolyte.
From www.animalia-life.club
Electrolyte Strength Chart Table Salt Strong Electrolyte Likewise, a strong acid like hcl splits. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. The word electrolyte generally refers to the largest concentration of charged particles within the human body. Electrolytes and sea salt vs table salt. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. (the. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID4763782 Table Salt Strong Electrolyte Electrolytes and sea salt vs table salt. (the biggest two are sodium. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. Strong electrolytes completely ionize when dissolved, and. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID7074703 Table Salt Strong Electrolyte Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. A strong electrolyte, like nacl, splits up completely into sodium and chloride ions in solution. The word electrolyte generally refers to the largest concentration of charged particles within the human body. Salt is actually a combination of two key electrolytes — sodium and chloride. Electrolytes and sea salt vs table. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download Table Salt Strong Electrolyte Salt is actually a combination of two key electrolytes — sodium and chloride. (the biggest two are sodium. For example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Electrolytes and sea salt vs table salt. The word electrolyte generally refers to the largest concentration of charged particles within the human body. Likewise, a strong acid like hcl splits. Electrolyte strength (est) in aqueous solutions, an. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Chemical reactions PowerPoint Presentation, free download ID Table Salt Strong Electrolyte (the biggest two are sodium. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Electrolytes and sea salt vs table salt. Those elements are among the list of substances classified as electrolytes. Likewise, a strong acid like hcl splits. Salt is actually a combination of two key electrolytes — sodium and chloride. A strong electrolyte, like nacl, splits up. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Table Salt Strong Electrolyte Those elements are among the list of substances classified as electrolytes. Salt is actually a combination of two key electrolytes — sodium and chloride. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value. Table Salt Strong Electrolyte.
From slideplayer.com
Chapter 4 Reactions in Aqueous Solution ppt download Table Salt Strong Electrolyte Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. Standard issue table salt contains both sodium and chloride and (consumed in moderation) is a great way to up the hydration value of your electrolyte beverage. (the biggest two are sodium. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. A strong electrolyte, like nacl,. Table Salt Strong Electrolyte.
From www.slideserve.com
PPT Unit VI Acids, Bases & Salts PowerPoint Presentation, free Table Salt Strong Electrolyte Likewise, a strong acid like hcl splits. Electrolyte strength (est) in aqueous solutions, an electrolyte dissociates into. Salt is actually a combination of two key electrolytes — sodium and chloride. Electrolytes and sea salt vs table salt. Those elements are among the list of substances classified as electrolytes. (the biggest two are sodium. A strong electrolyte, like nacl, splits up. Table Salt Strong Electrolyte.
From www.kentchemistry.com
Electrolytes Table Salt Strong Electrolyte Those elements are among the list of substances classified as electrolytes. Electrolytes and sea salt vs table salt. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. The word electrolyte generally refers to the largest concentration of charged particles within the human body. Standard issue table salt contains both sodium and chloride and (consumed in. Table Salt Strong Electrolyte.