Why Does Water Cool Down . The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: If the container is copper, for example, then the water will cool a lot faster than if the. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. 1) the thermal conductivity of the material from which the water container is made. The specific heat capacity of water is 4200 j/kg/c. Water cools down and heats up at exactly the same rate under ideal conditions. Millions of tons of water are cooled this way everyday in power plants world wide: Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The cooling towers drop hottish water from the top.
from fastlovehomes.com.au
Millions of tons of water are cooled this way everyday in power plants world wide: The cooling towers drop hottish water from the top. If the container is copper, for example, then the water will cool a lot faster than if the. The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. 1) the thermal conductivity of the material from which the water container is made. Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. Water cools down and heats up at exactly the same rate under ideal conditions. The specific heat capacity of water is 4200 j/kg/c.
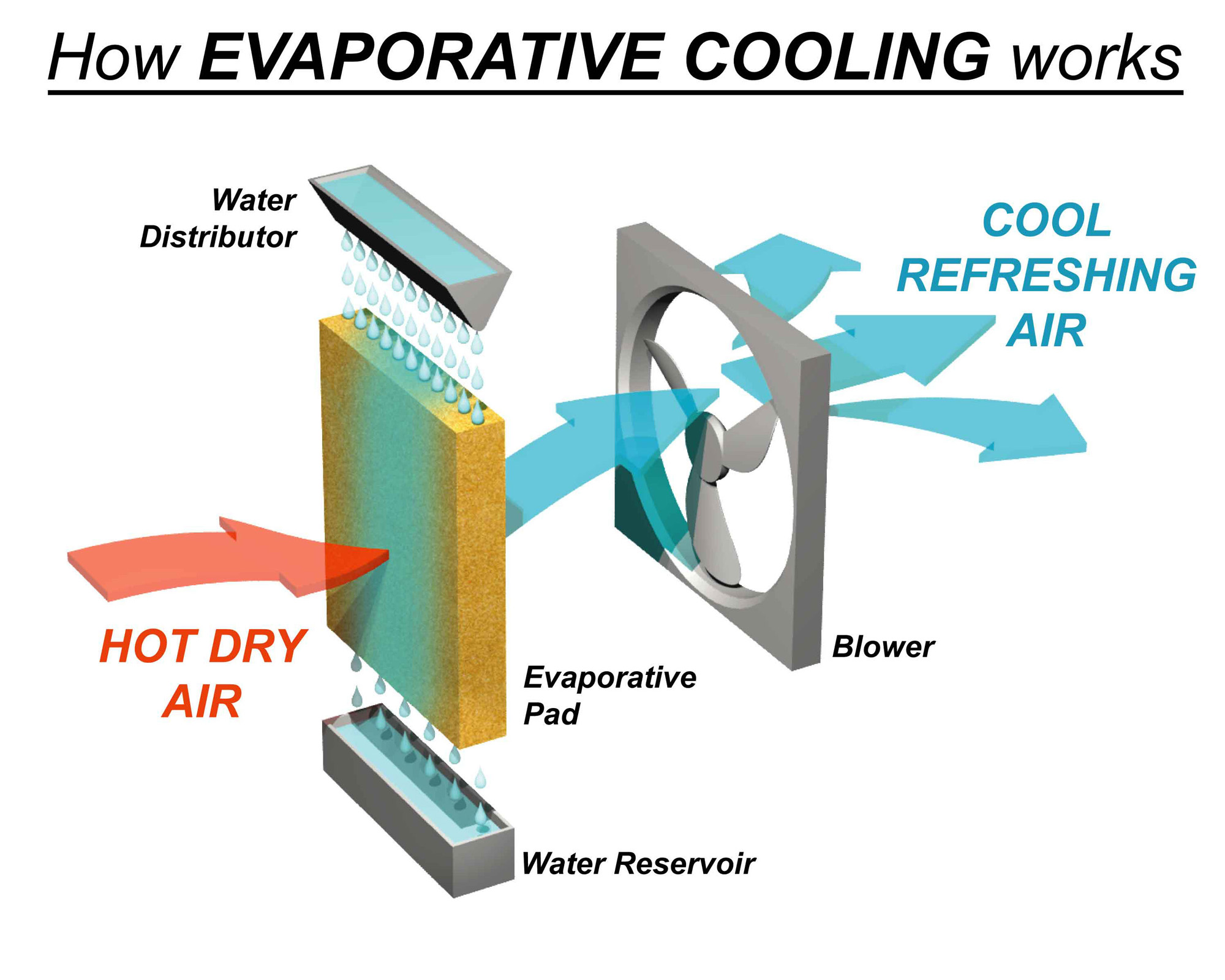
Split System vs Evaporative Cooling What is the difference?
Why Does Water Cool Down 1) the thermal conductivity of the material from which the water container is made. Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: 1) the thermal conductivity of the material from which the water container is made. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. Water cools down and heats up at exactly the same rate under ideal conditions. If the container is copper, for example, then the water will cool a lot faster than if the. The cooling towers drop hottish water from the top. Millions of tons of water are cooled this way everyday in power plants world wide: The specific heat capacity of water is 4200 j/kg/c.
From www.pinterest.com
COLD WATER 💦 VS HOT WATER 🔥 Throughout time, people have recognized Why Does Water Cool Down Water cools down and heats up at exactly the same rate under ideal conditions. The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: The specific heat capacity of water is 4200 j/kg/c. 1) the thermal conductivity of the material from which the water container is made. If. Why Does Water Cool Down.
From www.youtube.com
Water Cooling Explained How It Works and What Parts You Need YouTube Why Does Water Cool Down The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: The cooling towers drop hottish water from the top. Millions of tons of water are cooled this way everyday in power plants world wide: Water freezes from the top down—which allows ice to float—because of a strange quirk. Why Does Water Cool Down.
From www.youtube.com
Why evaporation is a cooling process? YouTube Why Does Water Cool Down Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. 1) the thermal conductivity of the material from which the water container is made. Water cools down and heats up at exactly the same rate under ideal conditions. The specific heat capacity of water is 4200 j/kg/c. The. Why Does Water Cool Down.
From www.researchgate.net
Assembly drawing of watercooled radiator of cooling tower in thermal Why Does Water Cool Down If the container is copper, for example, then the water will cool a lot faster than if the. The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: The specific heat capacity of water is 4200 j/kg/c. Water freezes from the top down—which allows ice to float—because of. Why Does Water Cool Down.
From owlcation.com
Should Water Be Used as a Refrigerant? Owlcation Why Does Water Cool Down The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. Water cools down and heats up at exactly the same rate under ideal conditions. 1). Why Does Water Cool Down.
From foodandfizz.com
How Fast Does Water Cool After Boiling? 1 Concrete Answer Why Does Water Cool Down The specific heat capacity of water is 4200 j/kg/c. Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. If the container is copper, for example, then the water will cool a lot faster than if the. Water freezes from the top down—which allows ice to float—because. Why Does Water Cool Down.
From www.mechanicalbooster.com
How Engine Cooling System Works? Mechanical Booster Why Does Water Cool Down The cooling towers drop hottish water from the top. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. Millions of tons of water are. Why Does Water Cool Down.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo Why Does Water Cool Down Millions of tons of water are cooled this way everyday in power plants world wide: Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. 1) the thermal conductivity of the material from which the water container is made. If the container is copper, for example, then the. Why Does Water Cool Down.
From www.youtube.com
How to keep Water Cool in Matka Evaporation in Earthen pot Pot Why Does Water Cool Down 1) the thermal conductivity of the material from which the water container is made. Millions of tons of water are cooled this way everyday in power plants world wide: The specific heat capacity of water is 4200 j/kg/c. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures.. Why Does Water Cool Down.
From www.shutterstock.com
Cool Down Cooling Off Concept Diver Stock Illustration 275272916 Why Does Water Cool Down Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The cooling towers drop hottish water from the top. Water cools down and heats up at exactly the same rate under ideal conditions. 1) the thermal conductivity of the material from which the water container is made.. Why Does Water Cool Down.
From www.pinterest.com
Cool Down, It's Just Water! Science education, Science, Education Why Does Water Cool Down If the container is copper, for example, then the water will cool a lot faster than if the. Millions of tons of water are cooled this way everyday in power plants world wide: The specific heat capacity of water is 4200 j/kg/c. 1) the thermal conductivity of the material from which the water container is made. The cooling towers drop. Why Does Water Cool Down.
From sciencenotes.org
Evaporative Cooler How It Works and Examples Why Does Water Cool Down Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The specific heat capacity of water is 4200 j/kg/c. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. The amount of thermal energy in. Why Does Water Cool Down.
From loricchandlero.blob.core.windows.net
How Do Evaporative Cooling Systems Work at loricchandlero blog Why Does Water Cool Down The cooling towers drop hottish water from the top. Water cools down and heats up at exactly the same rate under ideal conditions. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. Now, new research published on august 5 in the journal nature not only shows that. Why Does Water Cool Down.
From presswire18.com
Why does hot water cool down quickly? Do these 2 things quickly, you Why Does Water Cool Down If the container is copper, for example, then the water will cool a lot faster than if the. The cooling towers drop hottish water from the top. Millions of tons of water are cooled this way everyday in power plants world wide: The amount of thermal energy in the water is proportional to the volume of water (which is proportional. Why Does Water Cool Down.
From foodandfizz.com
How Fast Does Water Cool After Boiling? 1 Concrete Answer Why Does Water Cool Down The cooling towers drop hottish water from the top. Millions of tons of water are cooled this way everyday in power plants world wide: If the container is copper, for example, then the water will cool a lot faster than if the. Now, new research published on august 5 in the journal nature not only shows that the mpemba effect. Why Does Water Cool Down.
From www.watpure.com
Ways to cool down by drinking water Why Does Water Cool Down The cooling towers drop hottish water from the top. Millions of tons of water are cooled this way everyday in power plants world wide: Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. Water cools down and heats up at exactly the same rate under ideal conditions.. Why Does Water Cool Down.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Why Does Water Cool Down If the container is copper, for example, then the water will cool a lot faster than if the. The cooling towers drop hottish water from the top. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. Now, new research published on august 5 in the journal nature. Why Does Water Cool Down.
From jetcool.com
Water Cooling vs. Immersion Cooling for Bitcoin Mining JetCool Why Does Water Cool Down If the container is copper, for example, then the water will cool a lot faster than if the. The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: Water cools down and heats up at exactly the same rate under ideal conditions. The cooling towers drop hottish water. Why Does Water Cool Down.
From www.youtube.com
How fast does water cool off after boiling? YouTube Why Does Water Cool Down Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. The specific heat capacity of water is 4200 j/kg/c. If the container is copper, for example, then the water will cool a lot faster than if the. The cooling towers drop hottish water from the top. Millions of. Why Does Water Cool Down.
From bedcoolingsystem.s3.us-east-2.amazonaws.com
Why Does Water Cool Down At Night Spring Why Does Water Cool Down Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. Millions of tons of water are cooled this way everyday in power plants world wide: The specific heat capacity of water is 4200 j/kg/c. If the container is copper, for example, then the water will cool a. Why Does Water Cool Down.
From socalfresh.com
Why Water Is Cool So Cal Fresh Why Does Water Cool Down Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The specific heat capacity of water is 4200 j/kg/c. The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: The cooling towers drop hottish water. Why Does Water Cool Down.
From www.aplustopper.com
Expansion and Contraction in Solids, Liquids and Gases A Plus Topper Why Does Water Cool Down 1) the thermal conductivity of the material from which the water container is made. Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The specific heat capacity of water is 4200 j/kg/c. Water cools down and heats up at exactly the same rate under ideal conditions.. Why Does Water Cool Down.
From www.tffn.net
How Does Liquid Cooling Work? Exploring the Basics & Benefits of Liquid Why Does Water Cool Down Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The cooling towers drop hottish water from the top. If the container is copper, for example, then the water will cool a lot faster than if the. 1) the thermal conductivity of the material from which the. Why Does Water Cool Down.
From socratic.org
What happens to the energy of its molecules as ice melts into Why Does Water Cool Down The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The specific heat capacity of water is 4200 j/kg/c. If the container is copper, for. Why Does Water Cool Down.
From giodxpglk.blob.core.windows.net
How Long For Hot Water To Cool Down at Brandon Lewandowski blog Why Does Water Cool Down Millions of tons of water are cooled this way everyday in power plants world wide: The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds.. Why Does Water Cool Down.
From watercoolingsengihi.blogspot.com
Water Cooling Water Cooling Curve Why Does Water Cool Down Millions of tons of water are cooled this way everyday in power plants world wide: The cooling towers drop hottish water from the top. The specific heat capacity of water is 4200 j/kg/c. Water cools down and heats up at exactly the same rate under ideal conditions. The amount of thermal energy in the water is proportional to the volume. Why Does Water Cool Down.
From www.titanrig.com
Why To Water Cool Your PC & What Are The Benefits TITAN RIG Blog Why Does Water Cool Down Millions of tons of water are cooled this way everyday in power plants world wide: Water cools down and heats up at exactly the same rate under ideal conditions. If the container is copper, for example, then the water will cool a lot faster than if the. Now, new research published on august 5 in the journal nature not only. Why Does Water Cool Down.
From www.alamy.com
Spout with water, cool down and drink Stock Photo Alamy Why Does Water Cool Down 1) the thermal conductivity of the material from which the water container is made. The cooling towers drop hottish water from the top. Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. The specific heat capacity of water is 4200 j/kg/c. If the container is copper,. Why Does Water Cool Down.
From www.markedbyteachers.com
Investigate how the material of a cup affects the time it takes for the Why Does Water Cool Down The cooling towers drop hottish water from the top. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. 1) the thermal conductivity of the material from which the water container is made. If the container is copper, for example, then the water will cool a lot faster. Why Does Water Cool Down.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo Why Does Water Cool Down The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: If the container is copper, for example, then the water will cool a lot faster than if the. The specific heat capacity of water is 4200 j/kg/c. Water freezes from the top down—which allows ice to float—because of. Why Does Water Cool Down.
From www.teachoo.com
How does the water kept in an earthen pot (matka) cool during Why Does Water Cool Down The amount of thermal energy in the water is proportional to the volume of water (which is proportional to its size cubed: If the container is copper, for example, then the water will cool a lot faster than if the. Water cools down and heats up at exactly the same rate under ideal conditions. Water freezes from the top down—which. Why Does Water Cool Down.
From socratic.org
If you are wearing wet clothes, and the water evaporates, it cools you Why Does Water Cool Down 1) the thermal conductivity of the material from which the water container is made. Water cools down and heats up at exactly the same rate under ideal conditions. The specific heat capacity of water is 4200 j/kg/c. The cooling towers drop hottish water from the top. Now, new research published on august 5 in the journal nature not only shows. Why Does Water Cool Down.
From fastlovehomes.com.au
Split System vs Evaporative Cooling What is the difference? Why Does Water Cool Down Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. If the container is copper, for example, then the water will cool a lot faster. Why Does Water Cool Down.
From journal.uptimeinstitute.com
Ignore Data Center Water Consumption at Your Own Peril Uptime Why Does Water Cool Down Millions of tons of water are cooled this way everyday in power plants world wide: 1) the thermal conductivity of the material from which the water container is made. Water freezes from the top down—which allows ice to float—because of a strange quirk in how water’s density behaves at falling temperatures. The specific heat capacity of water is 4200 j/kg/c.. Why Does Water Cool Down.
From 2crsi.com
What Is Direct Liquid Cooling System? 2CRSi Why Does Water Cool Down 1) the thermal conductivity of the material from which the water container is made. Water cools down and heats up at exactly the same rate under ideal conditions. The cooling towers drop hottish water from the top. Now, new research published on august 5 in the journal nature not only shows that the mpemba effect does exist, but also sheds.. Why Does Water Cool Down.