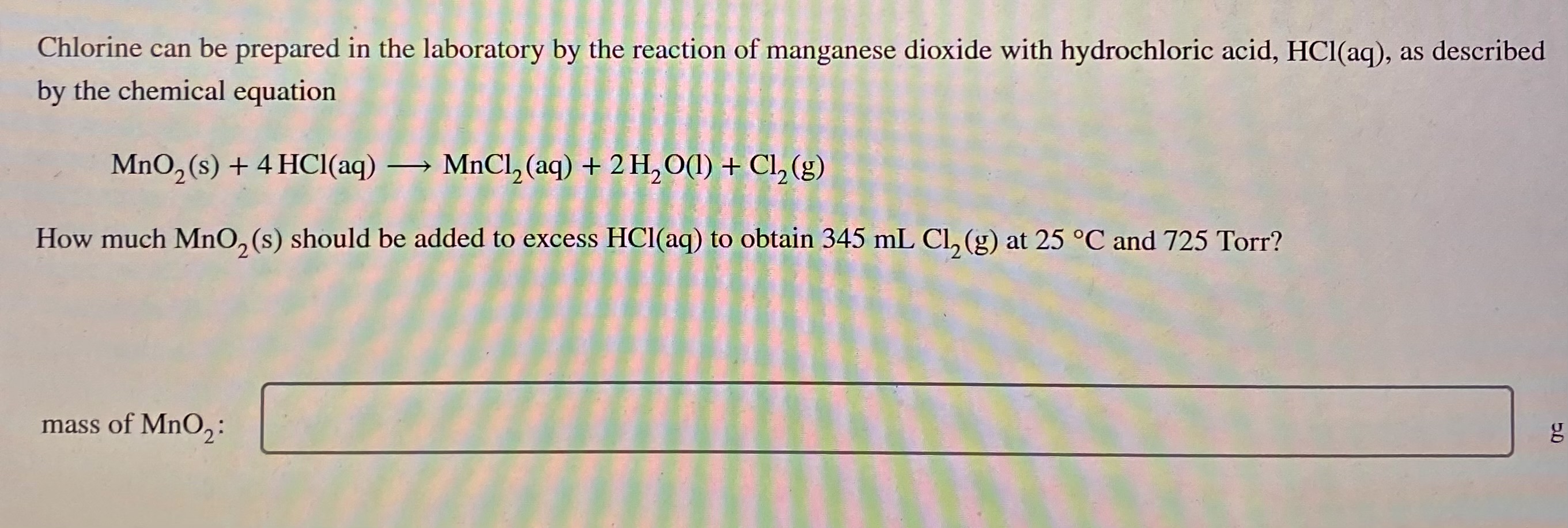How Can Chlorine Be Prepared In The Laboratory . Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. Its electron arrangement is 2.8.7 and it belongs to the. In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. This is the simplest method to prepare chlorine gas in the laboratory. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride:
from www.chegg.com
Its electron arrangement is 2.8.7 and it belongs to the. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). This is the simplest method to prepare chlorine gas in the laboratory. In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride:
Chlorine can be prepared in the laboratory by the
How Can Chlorine Be Prepared In The Laboratory This is the simplest method to prepare chlorine gas in the laboratory. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: This is the simplest method to prepare chlorine gas in the laboratory. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Its electron arrangement is 2.8.7 and it belongs to the. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction:
From www.numerade.com
SOLVED Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with How Can Chlorine Be Prepared In The Laboratory It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). This is the simplest method to prepare chlorine gas in the laboratory. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. In laboratories, chlorine is prepared from manganese dioxide (mno. How Can Chlorine Be Prepared In The Laboratory.
From www.coursehero.com
[Solved] Chlorine gas can be prepared in the laboratory by the reaction of... Course Hero How Can Chlorine Be Prepared In The Laboratory Its electron arrangement is 2.8.7 and it belongs to the. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. This is the simplest method to prepare chlorine. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. Its electron arrangement is 2.8.7 and it belongs to the. In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. The alternatives to kmno. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine gas can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory This is the simplest method to prepare chlorine gas in the laboratory. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Its electron arrangement is 2.8.7 and it. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine gas can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory This is the simplest method to prepare chlorine gas in the laboratory. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. Its electron arrangement is 2.8.7 and it belongs to the. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). In. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. This is the simplest method to prepare chlorine gas in the laboratory. Its electron arrangement is 2.8.7 and it belongs to the. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: In laboratories, chlorine is prepared from manganese dioxide (mno 2). How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: This is the simplest method to prepare chlorine gas in the laboratory. In laboratories, chlorine is prepared from manganese dioxide. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine gas can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Its electron arrangement is 2.8.7 and it belongs to the. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. The alternatives to kmno 4 are. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory This is the simplest method to prepare chlorine gas in the laboratory. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Its electron arrangement is 2.8.7 and it belongs to the. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl),. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine Can Be Prepared In The Laboratory By The How Can Chlorine Be Prepared In The Laboratory Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). This is the simplest method to prepare chlorine gas in the laboratory. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. In this method, potassium permagnate(kmno 4) is taken in a flask. How Can Chlorine Be Prepared In The Laboratory.
From www.youtube.com
Laboratory Preparation Of Chlorine Using Potassium Permanganate Explained! YouTube How Can Chlorine Be Prepared In The Laboratory This is the simplest method to prepare chlorine gas in the laboratory. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. In this method, potassium permagnate(kmno 4). How Can Chlorine Be Prepared In The Laboratory.
From www.bartleby.com
Answered Chlorine can be prepared in the… bartleby How Can Chlorine Be Prepared In The Laboratory In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Its electron arrangement is 2.8.7 and it belongs to the. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: This is the simplest method to prepare chlorine gas in the laboratory. In laboratories, chlorine is prepared from manganese dioxide (mno 2). How Can Chlorine Be Prepared In The Laboratory.
From www.fanpop.com
Preparation of chlorine (gas) in laboratory Chemistry Fanpop How Can Chlorine Be Prepared In The Laboratory Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). This is the simplest method to prepare chlorine. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). This is the simplest method to prepare chlorine gas in the laboratory. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. In this method, potassium permagnate(kmno 4) is taken in. How Can Chlorine Be Prepared In The Laboratory.
From www.youtube.com
Laboratory Preparation of Chlorine YouTube How Can Chlorine Be Prepared In The Laboratory Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Its electron arrangement is 2.8.7 and it belongs to the. In this method, potassium permagnate(kmno 4) is taken in a flask and. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). This is the simplest method to prepare chlorine gas in the laboratory. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid.. How Can Chlorine Be Prepared In The Laboratory.
From www.studypool.com
SOLUTION Solution for chlorine gas can be prepared in the laboratory by the reaction of How Can Chlorine Be Prepared In The Laboratory The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Its electron arrangement is 2.8.7 and it belongs to the. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine gas can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). Its electron arrangement is 2.8.7 and it belongs to the. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Laboratory preparation of chlorinechlorine is a highly reactive gas and does not.. How Can Chlorine Be Prepared In The Laboratory.
From www.youtube.com
Preparation of Chlorine gas in lab. diagram making video YouTube How Can Chlorine Be Prepared In The Laboratory The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Its electron arrangement is 2.8.7 and it belongs to the. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii).. How Can Chlorine Be Prepared In The Laboratory.
From www.coursehero.com
[Solved] 8. Chlorine gas can be prepared in the laboratory by the reaction... Course Hero How Can Chlorine Be Prepared In The Laboratory In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. Its electron arrangement is 2.8.7 and it belongs to the. Chorine can be prepared. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Laboratory preparation of chlorinechlorine is a highly. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved 5. Chlorine gas can be prepared in the laboratory by How Can Chlorine Be Prepared In The Laboratory Its electron arrangement is 2.8.7 and it belongs to the. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii).. How Can Chlorine Be Prepared In The Laboratory.
From buildupeducation.com
Laboratory method of preparation of hydrochloric acid How Can Chlorine Be Prepared In The Laboratory Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Its electron arrangement is 2.8.7 and it belongs to the. This is the simplest method to prepare chlorine gas in the laboratory. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). In. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Chemistry Archive July 06, 2016 How Can Chlorine Be Prepared In The Laboratory Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Its electron arrangement is 2.8.7 and it belongs to the. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. This is the simplest method to prepare chlorine gas in the laboratory.. How Can Chlorine Be Prepared In The Laboratory.
From www.coursehero.com
[Solved] Chlorine can be prepared in the laboratory by the reaction of... Course Hero How Can Chlorine Be Prepared In The Laboratory The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). Its electron arrangement is 2.8.7 and it belongs to the.. How Can Chlorine Be Prepared In The Laboratory.
From www.coursehero.com
[Solved] Chlorine can be prepared in the laboratory by the reaction of... Course Hero How Can Chlorine Be Prepared In The Laboratory In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). This is the simplest method to prepare chlorine gas in the laboratory. Laboratory preparation of chlorinechlorine. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine gas can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. This is the simplest method to prepare chlorine gas in the laboratory. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Laboratory preparation of. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. This. How Can Chlorine Be Prepared In The Laboratory.
From www.answersarena.com
[Solved] Chlorine gas can be prepared in the laboratory b How Can Chlorine Be Prepared In The Laboratory In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii). The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Laboratory preparation of chlorinechlorine is a highly. How Can Chlorine Be Prepared In The Laboratory.
From fr.dreamstime.com
Diagramme De Préparation Du Gaz De Chlorure D'hydrogène Illustration Stock Illustration du How Can Chlorine Be Prepared In The Laboratory The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. This is the simplest method to prepare chlorine gas in the laboratory. In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: In this method, potassium permagnate(kmno 4). How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory In laboratories, chlorine is prepared from manganese dioxide (mno 2) and hydrochloric acid (hcl), according to the following reaction: Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. This is the simplest method to prepare chlorine gas in the laboratory. Its electron. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can. be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory Its electron arrangement is 2.8.7 and it belongs to the. The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: This is the simplest method to prepare chlorine gas in the laboratory. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. Chorine can. How Can Chlorine Be Prepared In The Laboratory.
From www.toppr.com
Chlorine is prepared in the laboratory by treating manganese dioxide (MnO2) with aqueous How Can Chlorine Be Prepared In The Laboratory This is the simplest method to prepare chlorine gas in the laboratory. In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. Its electron arrangement is 2.8.7 and it belongs to the. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: Laboratory preparation of chlorinechlorine is a highly reactive gas and. How Can Chlorine Be Prepared In The Laboratory.
From www.numerade.com
⏩SOLVEDChlorine can be prepared in the laboratory by the reaction… Numerade How Can Chlorine Be Prepared In The Laboratory In this method, potassium permagnate(kmno 4) is taken in a flask and concentrated hydrochloric acid. This is the simplest method to prepare chlorine gas in the laboratory. Its electron arrangement is 2.8.7 and it belongs to the. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. It is conveniently prepared in the laboratory by acidifying the appropriate. How Can Chlorine Be Prepared In The Laboratory.
From www.chegg.com
Solved Chlorine can be prepared in the laboratory by the How Can Chlorine Be Prepared In The Laboratory Its electron arrangement is 2.8.7 and it belongs to the. It is conveniently prepared in the laboratory by acidifying the appropriate sodium chloride: The alternatives to kmno 4 are manganese (iv) oxide (mno 2),. Laboratory preparation of chlorinechlorine is a highly reactive gas and does not. Chorine can be prepared by oxidation of concentrated hydrochloric acid using potassium manganate (vii).. How Can Chlorine Be Prepared In The Laboratory.