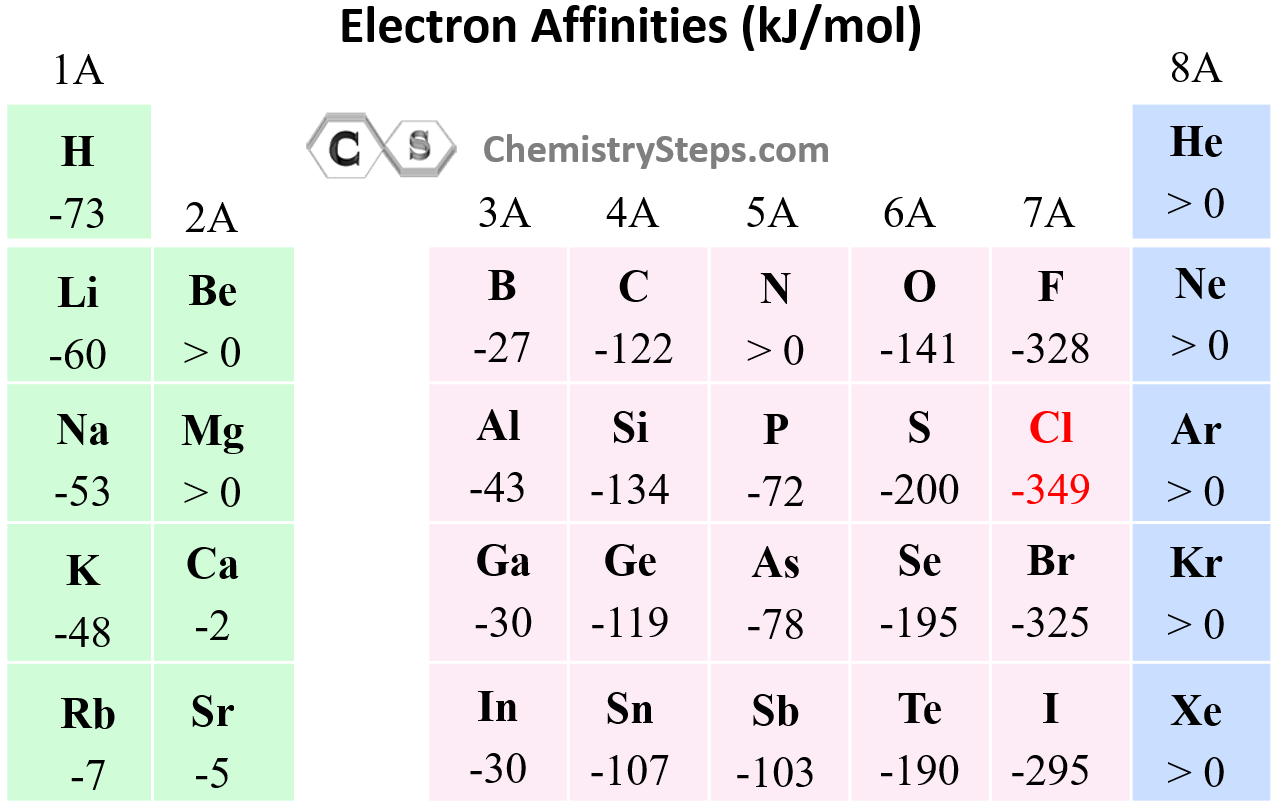
Bromine Electron Affinity Chlorine . Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how nuclear charge, distance and. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added.
from general.chemistrysteps.com
Learn how nuclear charge, distance and. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and.
Electron Affinity Chemistry Steps
Bromine Electron Affinity Chlorine Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how nuclear charge, distance and. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Electron affinity is the energy released when an atom gains an electron to form a negative ion.
From ar.inspiredpencil.com
Periodic Table With Electron Affinity Bromine Electron Affinity Chlorine Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. 125 rows find. Bromine Electron Affinity Chlorine.
From slideplayer.com
Learning Objectives General trends of group 17 elements ppt download Bromine Electron Affinity Chlorine Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how nuclear charge, distance and. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released. Bromine Electron Affinity Chlorine.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem Bromine Electron Affinity Chlorine Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how nuclear charge, distance and. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and. Bromine Electron Affinity Chlorine.
From ecurrencythailand.com
What Is The Electron Affinity Of Bromine? All Answers Bromine Electron Affinity Chlorine Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn how nuclear charge, distance and. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an. Bromine Electron Affinity Chlorine.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Affinity Chlorine Learn how nuclear charge, distance and. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Electron affinity is the energy released when an atom gains an electron to form a negative ion. 125 rows find the electron affinity values for various elements in the periodic table, defined as the. Bromine Electron Affinity Chlorine.
From loespjldd.blob.core.windows.net
Chlorine Electron Affinity Value at Timothy Peterson blog Bromine Electron Affinity Chlorine Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases. Bromine Electron Affinity Chlorine.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Bromine Electron Affinity Chlorine Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how the oxidising ability of the halogens (fluorine,. Bromine Electron Affinity Chlorine.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine Electron Affinity Chlorine Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. 125 rows find the electron affinity. Bromine Electron Affinity Chlorine.
From www.numerade.com
SOLVED While the electron affinity of bromine is a negative quantity Bromine Electron Affinity Chlorine Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn how nuclear charge, distance and. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and. Bromine Electron Affinity Chlorine.
From ceojpsva.blob.core.windows.net
Bromine Electron Dot Notation at Alana Jager blog Bromine Electron Affinity Chlorine Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn how nuclear charge,. Bromine Electron Affinity Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Bromine Electron Affinity Chlorine Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. 125 rows find the. Bromine Electron Affinity Chlorine.
From www.youtube.com
Halogens second electron Affinity is zero Why?Fluorine chlorine bromine Bromine Electron Affinity Chlorine Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn. Bromine Electron Affinity Chlorine.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Bromine Electron Affinity Chlorine 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn the definition,. Bromine Electron Affinity Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Bromine Electron Affinity Chlorine Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn how nuclear charge, distance and. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and.. Bromine Electron Affinity Chlorine.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Electron Affinity Chlorine Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn the definition, trends and. Bromine Electron Affinity Chlorine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Affinity Chlorine Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Electron affinity is the energy released when an atom gains an electron to form a negative ion. 125 rows find the electron affinity values for various. Bromine Electron Affinity Chlorine.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Bromine Electron Affinity Chlorine Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn. Bromine Electron Affinity Chlorine.
From dxorxejky.blob.core.windows.net
Does Chlorine Have A Larger Atomic Radius Than Fluorine at Leon Cain blog Bromine Electron Affinity Chlorine Learn how nuclear charge, distance and. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn. Bromine Electron Affinity Chlorine.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Bromine Electron Affinity Chlorine Learn how nuclear charge, distance and. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn the definition, trends and examples of electron affinity, the energy change. Bromine Electron Affinity Chlorine.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Bromine Electron Affinity Chlorine Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points,. Bromine Electron Affinity Chlorine.
From www.numerade.com
SOLVED Click the button that shows the correct relationship of the Bromine Electron Affinity Chlorine Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Learn how nuclear charge, distance and. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and. Bromine Electron Affinity Chlorine.
From www.numerade.com
SOLVED Does chlorine or bromine have a more negative (more favorable Bromine Electron Affinity Chlorine 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group.. Bromine Electron Affinity Chlorine.
From ecurrencythailand.com
Which Equation Corresponds To The Electron Affinity Of Chlorine? Top Bromine Electron Affinity Chlorine Learn how nuclear charge, distance and. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn the definition, trends and examples of electron affinity, the energy change. Bromine Electron Affinity Chlorine.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Bromine Electron Affinity Chlorine Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how nuclear charge, distance and. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius,. Bromine Electron Affinity Chlorine.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Bromine Electron Affinity Chlorine 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn how nuclear charge, distance and. Electron affinity is the energy released when an atom gains an electron. Bromine Electron Affinity Chlorine.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Bromine Electron Affinity Chlorine Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn how nuclear charge, distance and. 125 rows find the electron affinity values for various elements in the periodic table,. Bromine Electron Affinity Chlorine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Affinity Chlorine Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Learn how nuclear charge, distance and. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and. Bromine Electron Affinity Chlorine.
From byjus.com
6. d the electron affinity of chlorine from the following data Bromine Electron Affinity Chlorine Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Electron affinity is the energy released when an atom gains an electron to form a negative ion. 125 rows find the electron. Bromine Electron Affinity Chlorine.
From mavink.com
Periodic Table With Electron Affinity Bromine Electron Affinity Chlorine Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is. Bromine Electron Affinity Chlorine.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Electron Affinity Chlorine 125 rows find the electron affinity values for various elements in the periodic table, defined as the energy released when an electron is added. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how nuclear. Bromine Electron Affinity Chlorine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Bromine Electron Affinity Chlorine Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Learn how nuclear charge, distance and. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling. Bromine Electron Affinity Chlorine.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Bromine Electron Affinity Chlorine Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn. Bromine Electron Affinity Chlorine.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Electron Affinity Chlorine Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn how nuclear charge, distance and. Learn how the oxidising ability of the halogens (fluorine, chlorine, bromine and iodine) decreases as you go down the group. Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and. Bromine Electron Affinity Chlorine.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Bromine Electron Affinity Chlorine Learn about the five halogens (fluorine, chlorine, bromine, iodine, and astatine) and their characteristics, reactions, and. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. 125 rows find the electron. Bromine Electron Affinity Chlorine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Bromine Electron Affinity Chlorine Learn how the halogens (fluorine, chlorine, bromine and iodine) vary in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Electron affinity is the energy released when an atom gains an electron to form a negative ion. Learn about the. Bromine Electron Affinity Chlorine.