Why H2So4 Is Used In Titration . Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). \ce{naoh}\) is required to neutralize. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Hence sulfuric acid is stable in the present of strong oxidising.
from www.numerade.com
The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. \ce{naoh}\) is required to neutralize. Hence sulfuric acid is stable in the present of strong oxidising.
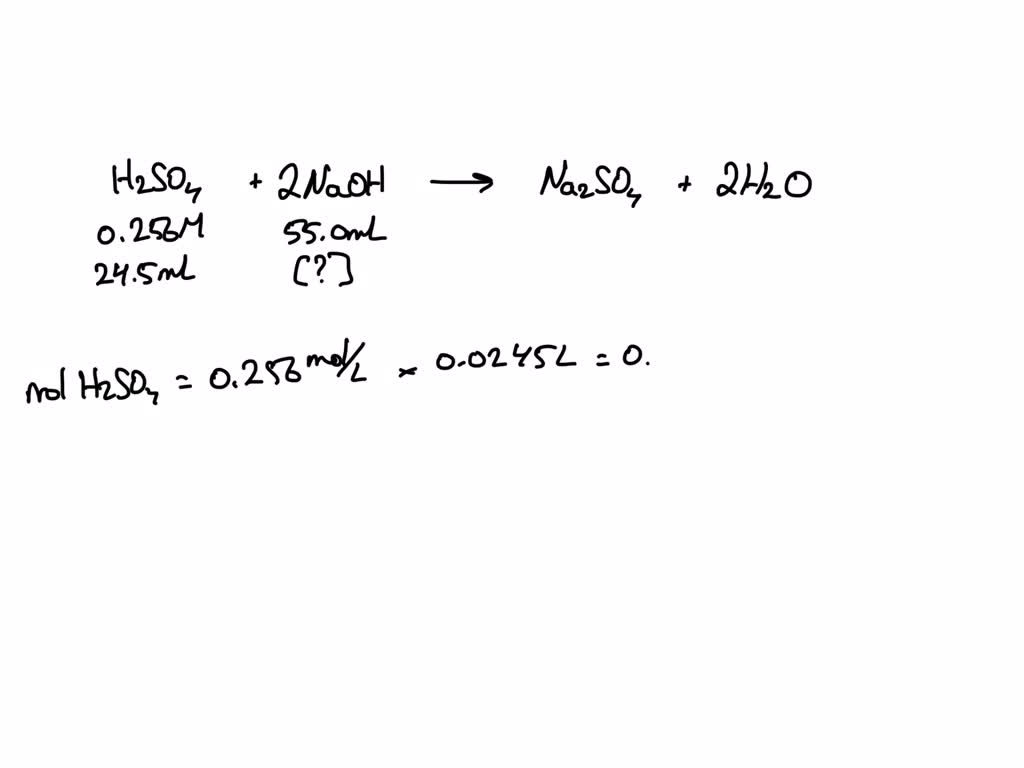
SOLVED A0.256 M of H2SO4 was used to titrate 55.0 ml of NaOH 24.5 ml
Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). \ce{naoh}\) is required to neutralize. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Hence sulfuric acid is stable in the present of strong oxidising. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \:
From www.numerade.com
A student carried out a titration using H2SO4 and KOH. The balanced Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Hence sulfuric acid is stable in the present of strong oxidising. H2so4. Why H2So4 Is Used In Titration.
From www.youtube.com
Titration Reaction H2SO4 and LiOH YouTube Why H2So4 Is Used In Titration Hence sulfuric acid is stable in the present of strong oxidising. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. H2so4. Why H2So4 Is Used In Titration.
From www.youtube.com
CHEM 1111 Lab 7 Determination of the Concentration of H2SO4 by Why H2So4 Is Used In Titration \ce{naoh}\) is required to neutralize. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Hence sulfuric acid is stable in the. Why H2So4 Is Used In Titration.
From www.numerade.com
SOLVED A0.256 M of H2SO4 was used to titrate 55.0 ml of NaOH 24.5 ml Why H2So4 Is Used In Titration The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. \ce{naoh}\) is required to neutralize. Hence sulfuric acid is stable in the present of strong oxidising. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and. Why H2So4 Is Used In Titration.
From slideplayer.com
Titrations!. ppt download Why H2So4 Is Used In Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. \ce{naoh}\) is required to neutralize. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these. Why H2So4 Is Used In Titration.
From oneclass.com
OneClass With .100 Molarity H2SO4 Use the titration results to Why H2So4 Is Used In Titration \ce{naoh}\) is required to neutralize. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. Hence sulfuric acid is stable in. Why H2So4 Is Used In Titration.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. Hence sulfuric acid is stable in the present of strong oxidising. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). In a titration of sulfuric acid against sodium hydroxide, \(32.20 \:. Why H2So4 Is Used In Titration.
From mavink.com
H2so4 Titration Curve Why H2So4 Is Used In Titration In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Hence sulfuric acid is stable in the present of strong oxidising. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). \ce{naoh}\). Why H2So4 Is Used In Titration.
From exokxncvu.blob.core.windows.net
Acid Base Titration Set Up at Eloy Owens blog Why H2So4 Is Used In Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which. Why H2So4 Is Used In Titration.
From www.chegg.com
Solved Titration of a 25.00 mL solution of H2SO4 requires Why H2So4 Is Used In Titration \ce{naoh}\) is required to neutralize. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark. Why H2So4 Is Used In Titration.
From www.numerade.com
SOLVED what is the function of H2SO4 in Redox Titration? why does we Why H2So4 Is Used In Titration \ce{naoh}\) is required to neutralize. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. Hence sulfuric acid is stable in the present of strong oxidising. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. In a titration of sulfuric acid against. Why H2So4 Is Used In Titration.
From dxoaiisdq.blob.core.windows.net
Titration Explained Simply at Marlene Barron blog Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: \ce{naoh}\) is required to neutralize. H2so4 increase the acidic content of the. Why H2So4 Is Used In Titration.
From www.numerade.com
SOLVED a titration was performed on a 25.0 mL sample of H2SO4 using 17 Why H2So4 Is Used In Titration \ce{naoh}\) is required to neutralize. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Hence sulfuric acid is stable in the present of strong oxidising. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid. Why H2So4 Is Used In Titration.
From www.numerade.com
SOLVED HNO3 and H2SO4 have the same molarity. Why did H2SO4 require Why H2So4 Is Used In Titration \ce{naoh}\) is required to neutralize. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Hence sulfuric acid is stable in the. Why H2So4 Is Used In Titration.
From klasiagdu.blob.core.windows.net
Titration Reactions Are Done To at Victoria Turney blog Why H2So4 Is Used In Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Hence sulfuric acid is stable in the present of strong oxidising. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Dilute. Why H2So4 Is Used In Titration.
From www.chegg.com
Solved A student performed three H2SO4 titrations in Part 2 Why H2So4 Is Used In Titration Hence sulfuric acid is stable in the present of strong oxidising. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. \ce{naoh}\). Why H2So4 Is Used In Titration.
From www.studypool.com
SOLUTION Solution for a student carried out a titration using h2so4 Why H2So4 Is Used In Titration In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hence sulfuric acid is stable in the present of strong oxidising. \ce{naoh}\) is required to neutralize. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere. Why H2So4 Is Used In Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Why H2So4 Is Used In Titration The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. H2so4 increase the acidic content of the solution so as to prevent. Why H2So4 Is Used In Titration.
From www.scribd.com
Acid Base Titration Lab H2so4 + Naoh AP Chem 2 Titration Chemistry Why H2So4 Is Used In Titration In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. The usual acid used in these situation is dilute $\ce{h2so4}$, which. Why H2So4 Is Used In Titration.
From slideplayer.com
Titrations!. ppt download Why H2So4 Is Used In Titration Hence sulfuric acid is stable in the present of strong oxidising. \ce{naoh}\) is required to neutralize. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Dilute sulfuric acid is added to prevent. Why H2So4 Is Used In Titration.
From www.coursehero.com
[Solved] A student performed three H2SO4 titrations in Part 2 using Why H2So4 Is Used In Titration In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Hence sulfuric acid is stable in the present of strong oxidising. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Dilute. Why H2So4 Is Used In Titration.
From byjus.com
Why is titration used? Why H2So4 Is Used In Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. \ce{naoh}\) is required to neutralize. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the. Why H2So4 Is Used In Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hence sulfuric acid is stable in the present of strong oxidising. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow).. Why H2So4 Is Used In Titration.
From www.coursehero.com
[Solved] A student performed three H2SO4 titrations in Part 2 using Why H2So4 Is Used In Titration H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of. Why H2So4 Is Used In Titration.
From www.scribd.com
Why We Use H2so4 in KMnO4 Titration PDF Titration Chemistry Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Hence sulfuric acid is stable in the present of strong oxidising. \ce{naoh}\). Why H2So4 Is Used In Titration.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Why H2So4 Is Used In Titration Hence sulfuric acid is stable in the present of strong oxidising. \ce{naoh}\) is required to neutralize. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere. Why H2So4 Is Used In Titration.
From www.coursehero.com
[Solved] For the titration of sulfuric acid (H2SO4) with sodium hyd Why H2So4 Is Used In Titration In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Hence sulfuric acid is stable in the present of strong oxidising. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. \ce{naoh}\). Why H2So4 Is Used In Titration.
From www.youtube.com
Identify hydrochloric acid and sulfuric acid solutions HCl and H2SO4 Why H2So4 Is Used In Titration In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hence sulfuric acid is stable in the present of strong oxidising. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. \ce{naoh}\). Why H2So4 Is Used In Titration.
From www.numerade.com
SOLVED For the titration reaction below H2SO4 + 2NaOH > 2H2O Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. Hence sulfuric acid is stable in the present of strong oxidising. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow).. Why H2So4 Is Used In Titration.
From mungfali.com
The Titration Of 25 0 Ml Of An Unknown Concentration Of H2so4 Solution 699 Why H2So4 Is Used In Titration \ce{naoh}\) is required to neutralize. H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Dilute sulfuric acid is added to prevent. Why H2So4 Is Used In Titration.
From exyouyqrf.blob.core.windows.net
Titration Apparatus And Uses at Nicole Fain blog Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. Hence sulfuric acid is stable in the present of strong oxidising. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow).. Why H2So4 Is Used In Titration.
From exoqwdiya.blob.core.windows.net
H2So4 Titration Graph at Jewell Hamilton blog Why H2So4 Is Used In Titration In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hence sulfuric acid is stable in the present of strong oxidising. \ce{naoh}\) is required to neutralize. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere. Why H2So4 Is Used In Titration.
From www.chegg.com
Solved Part 2 Data Table for H2SO4 titration Data Trial 1 Why H2So4 Is Used In Titration Hence sulfuric acid is stable in the present of strong oxidising. \ce{naoh}\) is required to neutralize. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere with the redox reaction. Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and. Why H2So4 Is Used In Titration.
From www.chegg.com
Solved A student carried out a titration using H2SO4 and Why H2So4 Is Used In Titration \ce{naoh}\) is required to neutralize. Hence sulfuric acid is stable in the present of strong oxidising. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: H2so4 increase the acidic content of the solution so as to prevent mno4 (purple) to reduced to mno2(dark brow). Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate). Why H2So4 Is Used In Titration.
From slideplayer.com
Christopher G. Hamaker, Illinois State University, Normal IL ppt download Why H2So4 Is Used In Titration Dilute sulfuric acid is added to prevent hydrolysis of mohr's salt (ferrous ammonium sulfate) and to maintain the acidic. Hence sulfuric acid is stable in the present of strong oxidising. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: \ce{naoh}\) is required to neutralize. The usual acid used in these situation is dilute $\ce{h2so4}$, which did not interfere. Why H2So4 Is Used In Titration.