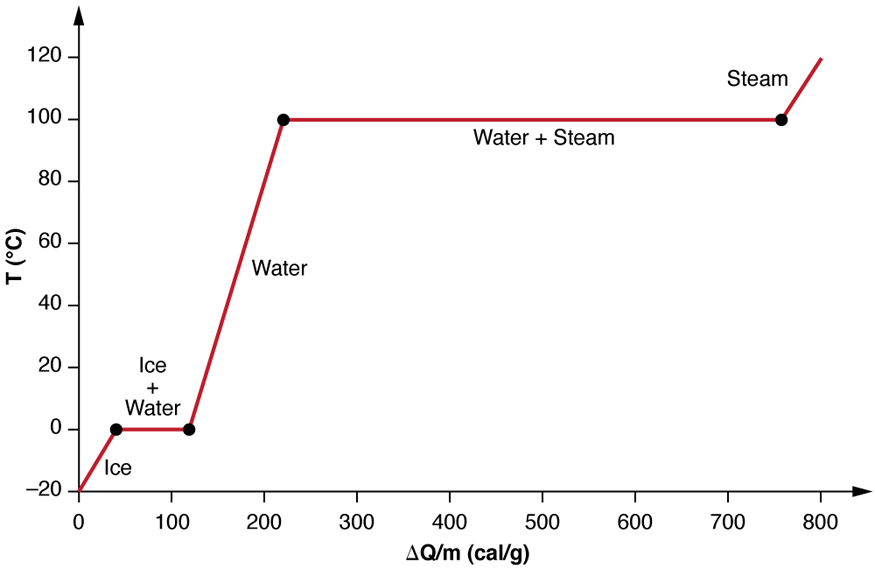
Heating Graph Physics . q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. — what happens to a substance when we heat it and it changes state? — the quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. These graphs can be used to understand.
from philschatz.com
— the quantitative relationship between heat transfer and temperature change contains all three factors: heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. — what happens to a substance when we heat it and it changes state? These graphs can be used to understand. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. Q = mcδt, where q is the. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.
Phase Change and Latent Heat · Physics
Heating Graph Physics These graphs can be used to understand. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. — what happens to a substance when we heat it and it changes state? learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. Q = mcδt, where q is the. These graphs can be used to understand. — the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams.
From www.studocu.com
Heating and cooling graph physics Heating and Cooling Graph For Exams Heating Graph Physics the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. q = mcδt, where q is the symbol for heat transfer (“quantity of. Heating Graph Physics.
From www.youtube.com
HTPIB14D5 Specific Heats from Graph YouTube Heating Graph Physics heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. These graphs can be used to understand. revision notes on 3.2.5 heating & cooling graphs for. Heating Graph Physics.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Pdf Heating Graph Physics — what happens to a substance when we heat it and it changes state? revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. — the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q. Heating Graph Physics.
From www.youtube.com
How to Read a Heating Curve YouTube Heating Graph Physics revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. — the quantitative relationship between heat transfer and temperature change contains all three factors: the specific heat. Heating Graph Physics.
From alevelphysicsnotes.com
Mr Toogood Physics Latent heat Heating Graph Physics — the quantitative relationship between heat transfer and temperature change contains all three factors: the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. Q =. Heating Graph Physics.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Graph Physics q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. revision notes on 3.2.5 heating & cooling graphs. Heating Graph Physics.
From ffden-2.phys.uaf.edu
Thermodynamics Heating Graph Physics revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. These graphs can be used to understand. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. heating and cooling graphs are graphs. Heating Graph Physics.
From spmphysics.onlinetuition.com.my
The Heating Curve SPM Physics Form 4/Form 5 Revision Notes Heating Graph Physics heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. — what happens to a substance when we heat it and it changes state?. Heating Graph Physics.
From ch301.cm.utexas.edu
heating curve Heating Graph Physics heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. Q = mcδt, where q is the. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus,. Heating Graph Physics.
From curiophysics.com
Heating Curve » Curio Physics Heating Graph Physics — what happens to a substance when we heat it and it changes state? heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt. Heating Graph Physics.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Graph Physics These graphs can be used to understand. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. — what happens to a substance when we heat it and it changes state?. Heating Graph Physics.
From www.youtube.com
Heating Curves Temperature Energy Graphs GCSE Physics YouTube Heating Graph Physics revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. These graphs can be used to understand. — what happens to a substance when we heat it and it changes state? learn about kinetic theory, which includes using the celsius and kelvin scales, the. Heating Graph Physics.
From www.showme.com
Topic Heating Curve ShowMe Online Learning Heating Graph Physics Q = mcδt, where q is the. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. These graphs can be used to understand. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. revision notes on 3.2.5 heating & cooling. Heating Graph Physics.
From philschatz.com
Phase Change and Latent Heat · Physics Heating Graph Physics Q = mcδt, where q is the. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. These graphs can be used to understand. — the quantitative relationship between heat transfer and temperature change contains all three factors: — what happens to a substance when we. Heating Graph Physics.
From www.excelatphysics.com
Heating Curve ExcelPhysics Heating Graph Physics These graphs can be used to understand. — the quantitative relationship between heat transfer and temperature change contains all three factors: learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00. Heating Graph Physics.
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Heating Graph Physics the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. — what happens to a substance when we heat it and it changes. Heating Graph Physics.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Graph Physics — the quantitative relationship between heat transfer and temperature change contains all three factors: learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. q = mcδt, where q. Heating Graph Physics.
From materialdbhutchins.z21.web.core.windows.net
Heat During Phase Change Formula Heating Graph Physics Q = mcδt, where q is the. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. — what happens to a substance when we heat it and it changes state? These graphs can be used to understand. learn about kinetic theory, which includes using the. Heating Graph Physics.
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Heating Graph Physics learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. These graphs can be used to understand. — what happens to a substance when we heat it and it changes state? q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the. Heating Graph Physics.
From study.com
Phase Changes and Heating Curves Lesson Heating Graph Physics These graphs can be used to understand. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. — what happens to a substance when we heat it and it changes state? Q = mcδt, where q is the. revision notes on 3.2.5 heating & cooling graphs. Heating Graph Physics.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Heating Graph Physics heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. — what happens to a substance when we heat it and it changes state? revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams.. Heating Graph Physics.
From gcsephysicsninja.com
49. Melting ice a heating graph Heating Graph Physics — the quantitative relationship between heat transfer and temperature change contains all three factors: — what happens to a substance when we heat it and it changes state? q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Q. Heating Graph Physics.
From www.sciencelearn.org.nz
Latent heat graph — Science Learning Hub Heating Graph Physics the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. — the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and. Heating Graph Physics.
From answerzoneschuster.z21.web.core.windows.net
What Is A Heat Curve Heating Graph Physics learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save. Heating Graph Physics.
From www.youtube.com
How to Read a Cooling Curve YouTube Heating Graph Physics heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. Q = mcδt, where q is the. the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. revision notes on 3.2.5 heating & cooling graphs for. Heating Graph Physics.
From study.com
Heating & Cooling Curves Definition, Phases & Examples Lesson Heating Graph Physics Q = mcδt, where q is the. These graphs can be used to understand. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. — the quantitative relationship between heat transfer and temperature change contains all three factors: learn about kinetic theory, which includes using the celsius. Heating Graph Physics.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Graph Physics These graphs can be used to understand. Q = mcδt, where q is the. revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. — what happens to a substance when we heat it and it changes state? the specific heat is the amount. Heating Graph Physics.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Graph Physics the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Q = mcδt, where q is the. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. q = mcδt, where q is the symbol for. Heating Graph Physics.
From www.knowledgeboat.com
(i) Define heat capacity of a substance. (ii) Write the SI KnowledgeBoat Heating Graph Physics heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. Q = mcδt, where q is the. — what happens to a substance when we heat it and it changes state? learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,.. Heating Graph Physics.
From exovqqtpg.blob.core.windows.net
What Should The Heat Be Set At at Beth Gray blog Heating Graph Physics revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. These graphs can be used to understand. — what happens to a substance when we heat it and it changes state? Q = mcδt, where q is the. q = mcδt, where q is. Heating Graph Physics.
From www.savemyexams.com
Heating & Cooling Graphs AQA GCSE Physics Revision Notes 2018 Heating Graph Physics — what happens to a substance when we heat it and it changes state? — the quantitative relationship between heat transfer and temperature change contains all three factors: These graphs can be used to understand. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. revision. Heating Graph Physics.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Heating Graph Physics learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. — what happens to a substance when we heat it and it changes state? q = mcδt, where q is. Heating Graph Physics.
From gcsephysicsninja.com
50. Heating ice and latent heat Heating Graph Physics heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. — what happens to a substance when we heat it and it changes state? the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. —. Heating Graph Physics.
From www.youtube.com
GCSE Physics Specific Latent Heat YouTube Heating Graph Physics revision notes on 3.2.5 heating & cooling graphs for the aqa gcse physics syllabus, written by the physics experts at save my exams. heating and cooling graphs are graphs that show how temperature changes over time as a result of heating or cooling. — what happens to a substance when we heat it and it changes state?. Heating Graph Physics.
From www.topperlearning.com
A piece of ice is heated at a constant rate. The variation in Heating Graph Physics — the quantitative relationship between heat transfer and temperature change contains all three factors: the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Q = mcδt, where q is the. heating and cooling graphs are graphs that show how temperature changes over time as a. Heating Graph Physics.