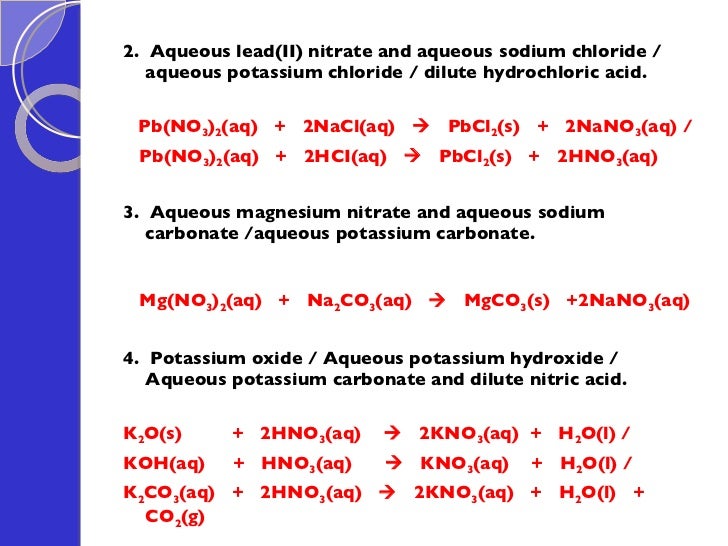
Potassium Sulfide And Zinc Chloride . When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. K 2 s + zncl 2 → 2kcl. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. The balanced equation will be calculated along with the. Reaction of potassium sulfide and zinc chloride. For example, ag3po4 + hcl. How many grams of zinc(ii) nitrate and sodium sulfide. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Enter an equation of an ionic chemical equation and press the balance button.
from hydrogenpotanezu.blogspot.com
When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. The balanced equation will be calculated along with the. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. For example, ag3po4 + hcl. How many grams of zinc(ii) nitrate and sodium sulfide. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. Enter an equation of an ionic chemical equation and press the balance button. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide :
Hydrogen Zinc Chloride Plus Hydrogen Sulfide
Potassium Sulfide And Zinc Chloride Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. Enter an equation of an ionic chemical equation and press the balance button. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Reaction of potassium sulfide and zinc chloride. For example, ag3po4 + hcl. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. How many grams of zinc(ii) nitrate and sodium sulfide. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. K 2 s + zncl 2 → 2kcl. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : The balanced equation will be calculated along with the.
From www.numerade.com
SOLVEDTABLE 5.45 Nume Cation Anion Compound cesium oxide Cst Csz0 potassium sulfide barium Potassium Sulfide And Zinc Chloride Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Reaction of potassium sulfide and zinc chloride. Enter an equation of an ionic chemical equation and press the balance button. How many grams of zinc(ii) nitrate and sodium sulfide. K 2 s + zncl 2 → 2kcl. Two important uses of precipitation reactions are to isolate metals. Potassium Sulfide And Zinc Chloride.
From www.toppr.com
10 fa) Write the electrondot structures potassium and chlorine. 16 Show the formation of KCl by Potassium Sulfide And Zinc Chloride Reaction of potassium sulfide and zinc chloride. K 2 s + zncl 2 → 2kcl. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : When zinc chloride (zncl2) is added to a. Potassium Sulfide And Zinc Chloride.
From www.chegg.com
Solved solution A solution B Does a precipitate form when A Potassium Sulfide And Zinc Chloride Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. The balanced equation will be calculated along with the. K 2 s + zncl 2 → 2kcl. When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVED Write the formulas for the following ionic compounds in which the metals have fixed Potassium Sulfide And Zinc Chloride Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. K 2 s + zncl 2 → 2kcl. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVED solution A solution B Does a precipitate form when A and B are mixed? empirical formula Potassium Sulfide And Zinc Chloride Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. K 2 s + zncl 2 → 2kcl. For example, ag3po4 + hcl. How many. Potassium Sulfide And Zinc Chloride.
From www.alamy.com
K2S potassium sulfide CAS 1312738 chemical substance in white plastic laboratory packaging Potassium Sulfide And Zinc Chloride Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. For example, ag3po4 + hcl.. Potassium Sulfide And Zinc Chloride.
From www.nanochemazone.com
Potassium Sulfide Powder Low Price 1 Nanochemazone Potassium Sulfide And Zinc Chloride When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. For example, ag3po4 + hcl. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVEDFeCl; iron(Il) chloride Hg(NO,h mercury(II) nitrite potassium sulfate KSO hospherue(I Potassium Sulfide And Zinc Chloride How many grams of zinc(ii) nitrate and sodium sulfide. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. For example, ag3po4 + hcl. Enter an equation of an ionic chemical equation and press the balance button. K 2 s + zncl 2 → 2kcl. Potassium. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVED '14. Arsenic (III) oxide reacts with hydrochloric acid. 15 . Potassium chromate reacts Potassium Sulfide And Zinc Chloride How many grams of zinc(ii) nitrate and sodium sulfide. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. When zinc chloride (zncl2) is added to a solution of potassium. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVED Data Table 2. Write the Formulas of the Following Compounds Compound Name potassium Potassium Sulfide And Zinc Chloride Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. For example, ag3po4 + hcl. K 2 s + zncl 2 → 2kcl. When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. Reaction of. Potassium Sulfide And Zinc Chloride.
From www.coursehero.com
[Solved] The chemical formula for potassium sulfide is KSO 3 OK2503 OK2s O KS2 Course Hero Potassium Sulfide And Zinc Chloride Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. The balanced equation will be calculated along with the. Potassium sulfide + zinc chloride =. Potassium Sulfide And Zinc Chloride.
From www.fishersci.com
Strem, An Ascensus Company CAS 1312738. 5g. Potassium sulfide, anhydrous, Fisher Scientific Potassium Sulfide And Zinc Chloride Reaction of potassium sulfide and zinc chloride. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. K 2 s + zncl 2 →. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVED Does Silver Nitrate and Potassium Sulfide precipitate when mixed? If so what is the Potassium Sulfide And Zinc Chloride A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Reaction of potassium sulfide and zinc chloride. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Adding 10.0 ml of a dilute solution of zinc nitrate. Potassium Sulfide And Zinc Chloride.
From slideplayer.com
Naming Polyatomic Ions ppt download Potassium Sulfide And Zinc Chloride When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. Reaction of potassium sulfide and zinc chloride. How many grams of zinc(ii) nitrate and sodium sulfide. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. For example,. Potassium Sulfide And Zinc Chloride.
From slideplayer.com
10/23/09 Naming Compounds Day ppt download Potassium Sulfide And Zinc Chloride A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. The balanced equation will be calculated along with the. Reaction of potassium sulfide and zinc chloride. How many grams of. Potassium Sulfide And Zinc Chloride.
From www.chegg.com
Solved Give the formula for the following ionic compounds. Potassium Sulfide And Zinc Chloride Reaction of potassium sulfide and zinc chloride. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : The balanced equation will be calculated along with the. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. A typical precipitation reaction occurs when an. Potassium Sulfide And Zinc Chloride.
From www.slideserve.com
PPT Double Replacement 1. Hydrogen sulfide is bubbled through a solution of silver nitrate Potassium Sulfide And Zinc Chloride The balanced equation will be calculated along with the. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. How many grams of zinc(ii) nitrate and sodium sulfide. Enter an equation of an ionic chemical equation and press the balance button. Adding 10.0 ml of a dilute solution of. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVEDIf aqueous solutions of potassium sulfide and iron(III) chloride are mixed, a precipitate Potassium Sulfide And Zinc Chloride The balanced equation will be calculated along with the. How many grams of zinc(ii) nitrate and sodium sulfide. Reaction of potassium sulfide and zinc chloride. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Enter. Potassium Sulfide And Zinc Chloride.
From animalia-life.club
Potassium Chloride Lewis Dot Structure Potassium Sulfide And Zinc Chloride Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. K 2 s + zncl 2 → 2kcl. For example, ag3po4 + hcl. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Potassium sulfide + zinc chloride = 2. Potassium Sulfide And Zinc Chloride.
From ar.inspiredpencil.com
Kcl Compound Potassium Sulfide And Zinc Chloride For example, ag3po4 + hcl. Enter an equation of an ionic chemical equation and press the balance button. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. The balanced equation will be calculated along with. Potassium Sulfide And Zinc Chloride.
From slideplayer.com
WRITING AND NAMING IONIC COMPOUNDS ppt download Potassium Sulfide And Zinc Chloride When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. Reaction of potassium sulfide and zinc chloride. For example, ag3po4 + hcl. K 2 s + zncl 2 → 2kcl. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVED When a solution of Cu(II) chloride, CuCl2, is added to a solution of potassium sulfide Potassium Sulfide And Zinc Chloride K 2 s + zncl 2 → 2kcl. Reaction of potassium sulfide and zinc chloride. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Enter an. Potassium Sulfide And Zinc Chloride.
From favpng.com
Potassium Sulfide Chemistry Crystal Structure, PNG, 1100x1068px, Potassium Sulfide, Area Potassium Sulfide And Zinc Chloride Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for. Potassium Sulfide And Zinc Chloride.
From www.coursehero.com
[Solved] Give the formulas of the following compounds potassium sulfide... Course Hero Potassium Sulfide And Zinc Chloride When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. For example, ag3po4 + hcl. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Reaction of potassium sulfide and zinc chloride. How many grams. Potassium Sulfide And Zinc Chloride.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Potassium Sulfide And Zinc Chloride Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. The balanced equation will be calculated along with the. Reaction of potassium. Potassium Sulfide And Zinc Chloride.
From slideplayer.com
What’s in a name?. ppt download Potassium Sulfide And Zinc Chloride Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react. Potassium Sulfide And Zinc Chloride.
From www.chimicamo.org
Potassiumsulfideunitcell3Dionic Potassium Sulfide And Zinc Chloride Reaction of potassium sulfide and zinc chloride. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. For example, ag3po4 + hcl. Write the net ionic equation for the precipitation. Potassium Sulfide And Zinc Chloride.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Potassium Sulfide And Zinc Chloride Reaction of potassium sulfide and zinc chloride. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Enter an equation of an ionic chemical equation and press the balance button. When zinc chloride. Potassium Sulfide And Zinc Chloride.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UWMadison Chemistry 103/104 Potassium Sulfide And Zinc Chloride A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. Two important uses of precipitation reactions are. Potassium Sulfide And Zinc Chloride.
From www.slideserve.com
PPT Na 3 PO 4 + 3 KOH → 3 NaOH + K 3 PO 4 Double Displacement PowerPoint Presentation ID7100658 Potassium Sulfide And Zinc Chloride Reaction of potassium sulfide and zinc chloride. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. A typical precipitation reaction occurs when an aqueous. Potassium Sulfide And Zinc Chloride.
From hydrogenpotanezu.blogspot.com
Hydrogen Zinc Chloride Plus Hydrogen Sulfide Potassium Sulfide And Zinc Chloride The balanced equation will be calculated along with the. K 2 s + zncl 2 → 2kcl. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of zinc chloride and potassium sulfide are. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : For example, ag3po4 + hcl. When zinc chloride (zncl2). Potassium Sulfide And Zinc Chloride.
From www.guidechem.com
POTASSIUM SULFIDE 1312738 wiki Potassium Sulfide And Zinc Chloride Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Reaction of potassium sulfide and zinc chloride. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. K 2 s + zncl. Potassium Sulfide And Zinc Chloride.
From www.numerade.com
SOLVED Does a reaction occur when aqueous solutions of potassium sulfide and iron(II) chloride Potassium Sulfide And Zinc Chloride A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. How many grams of zinc(ii) nitrate and sodium sulfide. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : For example, ag3po4 + hcl. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions. Potassium Sulfide And Zinc Chloride.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Potassium Sulfide And Zinc Chloride Enter an equation of an ionic chemical equation and press the balance button. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : K 2 s + zncl 2 → 2kcl. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. How many grams of zinc(ii) nitrate and. Potassium Sulfide And Zinc Chloride.
From www.slideshare.net
Precipitates Potassium Sulfide And Zinc Chloride For example, ag3po4 + hcl. K 2 s + zncl 2 → 2kcl. How many grams of zinc(ii) nitrate and sodium sulfide. Potassium sulfide + zinc chloride = 2 potassium chloride + zinc sulfide : When zinc chloride (zncl2) is added to a solution of potassium sulfide (k2s), the two compounds react in an aqueous medium. Two important uses of. Potassium Sulfide And Zinc Chloride.