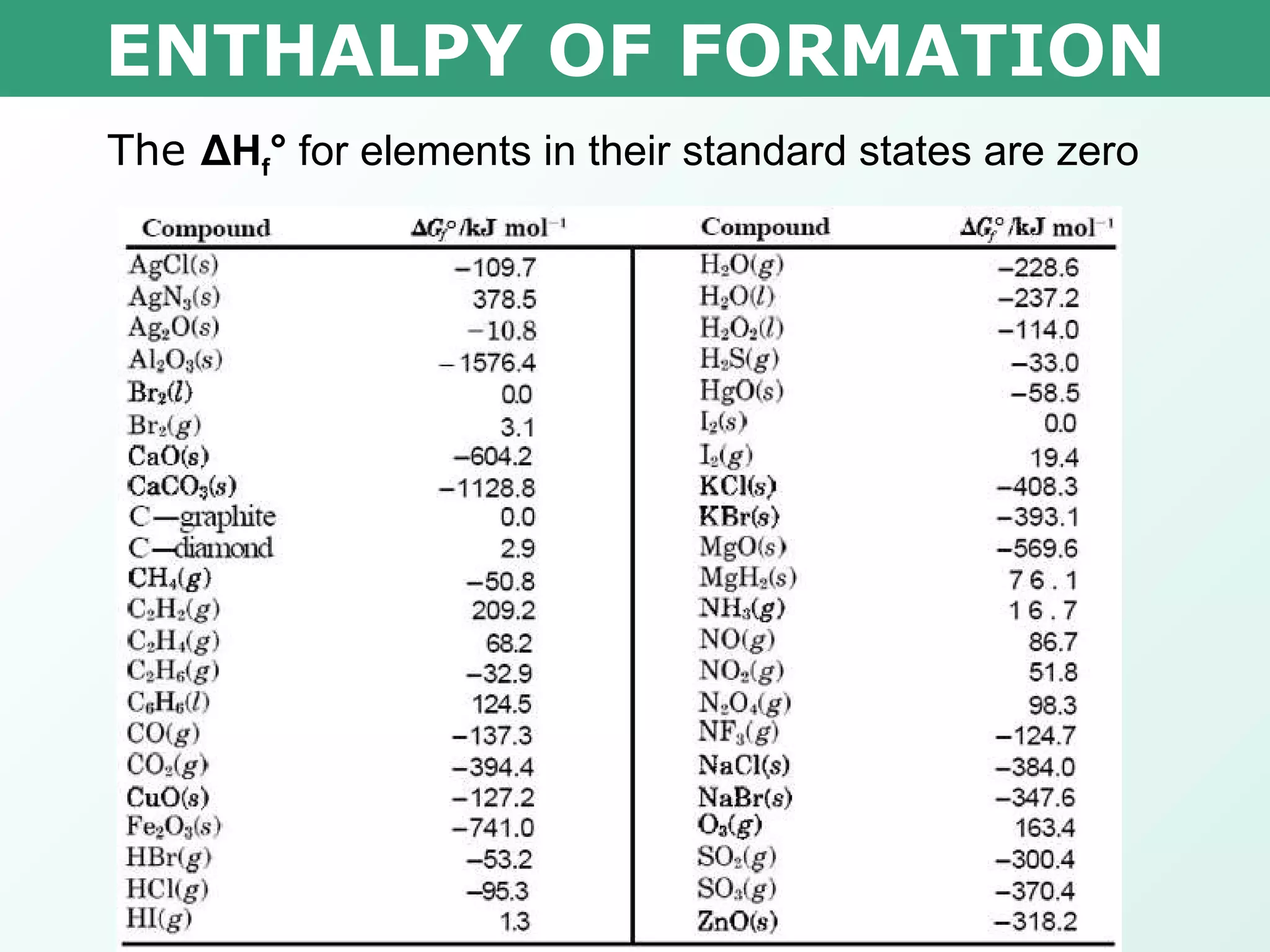
Standard Heat Of Formation And Combustion . The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: The key to solving this.
from www.slideshare.net
The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The key to solving this. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
Tang 03 enthalpy of formation and combustion PPT
Standard Heat Of Formation And Combustion The key to solving this. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The key to solving this. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation And Combustion 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of. Standard Heat Of Formation And Combustion.
From byjus.com
The heat of combustion of CH4(g),graphite and H2(g) are 20 kcal, 40 kcal and 10 kcal Standard Heat Of Formation And Combustion Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: The standard. Standard Heat Of Formation And Combustion.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation And Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The heat of formation or enthalpy of formation is the. Standard Heat Of Formation And Combustion.
From byjus.com
21. At 300 K standard enthalpy of formation of C6H5COOH(s), CO2(g), and H2O(l) are 408, 393, and Standard Heat Of Formation And Combustion The key to solving this. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is. Standard Heat Of Formation And Combustion.
From kaden-chapter.blogspot.com
Standard Enthalpy Of Formation Table Pdf 30+ Pages Summary [1.8mb] Latest Update Kaden Books Standard Heat Of Formation And Combustion The key to solving this. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and. Standard Heat Of Formation And Combustion.
From mungfali.com
Standard Heat Formation Chart Standard Heat Of Formation And Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The key to solving this. It is so. Standard Heat Of Formation And Combustion.
From byjus.com
The standard molar heat for formation ofethane, carbondioxide and water are respectively, 21.1 Standard Heat Of Formation And Combustion The key to solving this. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. 193 rows. Standard Heat Of Formation And Combustion.
From svanfitz.blogspot.com
Enthalpy Change Of Combustion PPT ENTHALPY OF FORMATION Combustion of Methanol The Standard Heat Of Formation And Combustion The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole. Standard Heat Of Formation And Combustion.
From printableliblayette.z13.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation And Combustion The key to solving this. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Standard enthalpy of combustion (δ h c °) (δ h c °). Standard Heat Of Formation And Combustion.
From byjus.com
43. Calculate standard heat of formation of CS2. Given that standard heat of combustion of C, S Standard Heat Of Formation And Combustion The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Heat Of Formation And Combustion.
From www.researchgate.net
Enthalpy of formation and heats of formation of main combustion... Download Scientific Diagram Standard Heat Of Formation And Combustion The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The key to solving this. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of formation is. Standard Heat Of Formation And Combustion.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Heat Of Formation And Combustion 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting. Standard Heat Of Formation And Combustion.
From www.chemistrystudent.com
Hess' Law and Hess Cycles (ALevel) ChemistryStudent Standard Heat Of Formation And Combustion Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: The heat of formation or enthalpy of. Standard Heat Of Formation And Combustion.
From maraferstravis.blogspot.com
Standard Enthalpy of Combustion Standard Heat Of Formation And Combustion Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Heat Of Formation And Combustion.
From byjus.com
under the same conditions heat of formation of water and CO2 are 285, 394 kj /mol the heat of Standard Heat Of Formation And Combustion The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: The key to solving this. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the. Standard Heat Of Formation And Combustion.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Heat Of Formation And Combustion The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1. Standard Heat Of Formation And Combustion.
From www.numerade.com
SOLVED a. Use standard heats of formation to determine the heat of combustion for the following Standard Heat Of Formation And Combustion 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying. Standard Heat Of Formation And Combustion.
From www.slideserve.com
PPT Energy changes PowerPoint Presentation, free download ID3003657 Standard Heat Of Formation And Combustion 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: The key to solving this. Standard enthalpy of combustion (δ h c °) (δ h c °) is. Standard Heat Of Formation And Combustion.
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion Standard Heat Of Formation And Combustion The key to solving this. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Standard enthalpy of formation is defined as the enthalpy change when. Standard Heat Of Formation And Combustion.
From www.slideserve.com
PPT ENTHALPY OF FORMATION Combustion of Methanol PowerPoint Presentation ID2263108 Standard Heat Of Formation And Combustion Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The key to. Standard Heat Of Formation And Combustion.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Presentation ID780619 Standard Heat Of Formation And Combustion The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Standard enthalpy of formation is defined as the enthalpy change. Standard Heat Of Formation And Combustion.
From www.youtube.com
Enthalpy of combustion hRP YouTube Standard Heat Of Formation And Combustion The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. It is so common that the phrase standard enthalpy of combustion is used alot and is given this. Standard Heat Of Formation And Combustion.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID2429720 Standard Heat Of Formation And Combustion The key to solving this. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. It is so common that the phrase standard enthalpy of combustion is used alot and is. Standard Heat Of Formation And Combustion.
From maraferstravis.blogspot.com
Standard Enthalpy of Combustion Standard Heat Of Formation And Combustion The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole. Standard Heat Of Formation And Combustion.
From byjus.com
Calculate standard heat of combustion of ethanol(C2H5OH(I)). Given that A,H (C2H5OH, I) 278 kJ Standard Heat Of Formation And Combustion The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound.. Standard Heat Of Formation And Combustion.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of formation and standard enthalpy Standard Heat Of Formation And Combustion The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole. Standard Heat Of Formation And Combustion.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion PPT Standard Heat Of Formation And Combustion 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The heat of formation or enthalpy of formation is the standard. Standard Heat Of Formation And Combustion.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Heat Of Formation And Combustion Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Heat Of Formation And Combustion.
From www.slideserve.com
PPT Energetics PowerPoint Presentation ID1204656 Standard Heat Of Formation And Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. It is so common that the phrase standard enthalpy of combustion is used alot and is. Standard Heat Of Formation And Combustion.
From www.youtube.com
15.1.1 Define Standard State, Enthalpy change of formation and combustion IB Chemistry HL YouTube Standard Heat Of Formation And Combustion The key to solving this. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. 193 rows. Standard Heat Of Formation And Combustion.
From www.youtube.com
STANDARD ENTHALPY OF COMBUSTION, FORMATION, HEAT OF DILUTION AND HEAT OF SOLUTION YouTube Standard Heat Of Formation And Combustion The key to solving this. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. It is so common that the phrase standard. Standard Heat Of Formation And Combustion.
From www.chegg.com
Solved how do I find standard heat of formation using Standard Heat Of Formation And Combustion It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: The key to solving this. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Heat Of Formation And Combustion.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Combustion of Methane Using Standard Heat Of Formation And Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Heat Of Formation And Combustion.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Heat Of Formation And Combustion The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of formation is defined as the. Standard Heat Of Formation And Combustion.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion PPT Standard Heat Of Formation And Combustion The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The key to solving this. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Heat Of Formation And Combustion.