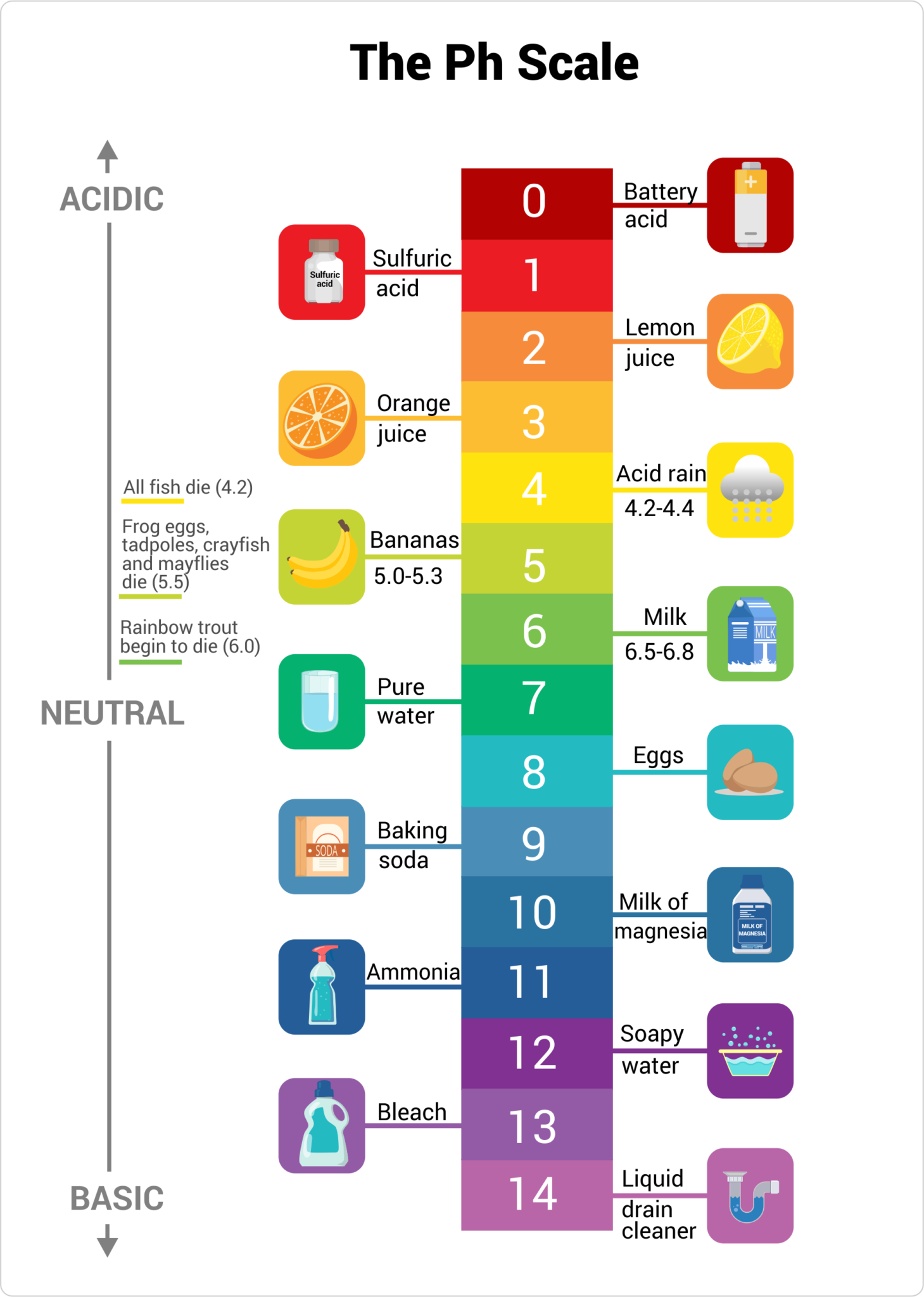
How To Measure Ph Scale . The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. A value less than 7 indicates that. Tell the origin and the logic of using the ph scale. There are multiple methods of measuring ph. For example, a ph of 3 is ten times more acidic than a. The range of ph goes from 0 to 14. Apply the same strategy for representing other types of. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in. To define the ph scale as a measure of acidity of a solution; When pure water is dropped into a solution of universal indicator, the. Ph is measured on a logarithmic scale. Ph represents the concentration of hydrogen ions (h+) in a solution and is. In this article, we explore the diverse methods and techniques available for ph measurement.
from www.mometrix.com
In this article, we explore the diverse methods and techniques available for ph measurement. Ph represents the concentration of hydrogen ions (h+) in a solution and is. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. A value less than 7 indicates that. When pure water is dropped into a solution of universal indicator, the. There are multiple methods of measuring ph. Ph is measured on a logarithmic scale. To define the ph scale as a measure of acidity of a solution; Tell the origin and the logic of using the ph scale.
pH Overview (Chemistry Review Video)
How To Measure Ph Scale For example, a ph of 3 is ten times more acidic than a. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. For example, a ph of 3 is ten times more acidic than a. Ph is measured on a logarithmic scale. There are multiple methods of measuring ph. The range of ph goes from 0 to 14. Ph represents the concentration of hydrogen ions (h+) in a solution and is. To define the ph scale as a measure of acidity of a solution; Tell the origin and the logic of using the ph scale. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. A value less than 7 indicates that. In this article, we explore the diverse methods and techniques available for ph measurement. Apply the same strategy for representing other types of. When pure water is dropped into a solution of universal indicator, the. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in. The formula for ph is.
From commons.wikimedia.org
File2713 pH Scale01.jpg How To Measure Ph Scale Ph is measured on a logarithmic scale. The formula for ph is. A value less than 7 indicates that. Apply the same strategy for representing other types of. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Since many reactions in everyday life are affected by. How To Measure Ph Scale.
From www.alamy.com
Measurement of the pH scale, pressure gauge, infographics. pH is a How To Measure Ph Scale For example, a ph of 3 is ten times more acidic than a. Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. Ph represents the concentration of hydrogen ions (h+) in a solution and is. The ph scale is logarithmic, meaning that an increase or decrease of an integer. How To Measure Ph Scale.
From sciencesummative.wordpress.com
pH Scale sciencesummative How To Measure Ph Scale The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Tell the origin and the logic of using the ph scale. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium. How To Measure Ph Scale.
From iwaterpurification.com
The pH Scale How To Measure Ph Scale Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. Tell the origin and the logic of using the ph scale. The range of ph goes from 0 to 14. When pure water is dropped into a solution of universal indicator, the. Ph represents the concentration of hydrogen ions (h+). How To Measure Ph Scale.
From www.mometrix.com
pH Overview (Chemistry Review Video) How To Measure Ph Scale In this article, we explore the diverse methods and techniques available for ph measurement. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in. For example, a ph of 3 is ten times more acidic than a. The formula for. How To Measure Ph Scale.
From www.pinterest.com
pH Scale Definition, Measurement, & Facts in 2021 Nomenclature How To Measure Ph Scale To define the ph scale as a measure of acidity of a solution; One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in. There are multiple methods of measuring ph. Since many reactions in everyday life are affected by ph,. How To Measure Ph Scale.
From sciencenotes.org
The pH Scale of Common Chemicals How To Measure Ph Scale The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. For example, a ph of 3 is ten times more acidic than a. There are multiple methods of measuring ph. A value less than 7 indicates that. Ph is measured on a logarithmic scale. When pure water. How To Measure Ph Scale.
From www.slideserve.com
PPT Understanding pH Scale and the Effects of Dilution on Acids and How To Measure Ph Scale Ph represents the concentration of hydrogen ions (h+) in a solution and is. The range of ph goes from 0 to 14. Ph is measured on a logarithmic scale. For example, a ph of 3 is ten times more acidic than a. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from. How To Measure Ph Scale.
From www.schoolofnaturalskincare.com
How to test and adjust the pH of natural skincare products (and why you How To Measure Ph Scale To define the ph scale as a measure of acidity of a solution; The range of ph goes from 0 to 14. For example, a ph of 3 is ten times more acidic than a. Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. The formula for ph is.. How To Measure Ph Scale.
From www.pmel.noaa.gov
The pH scale by numbers How To Measure Ph Scale Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. Apply the same strategy for representing other types of. For example, a ph of 3 is ten times more acidic than a. There are multiple methods of measuring ph. In this article, we explore the diverse methods and techniques available. How To Measure Ph Scale.
From www.worksheetsplanet.com
What is Ph Scale Definition of Ph Scale How To Measure Ph Scale Tell the origin and the logic of using the ph scale. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. To define the ph scale as a measure. How To Measure Ph Scale.
From www.youtube.com
MB.1.2. Ph scale (HSC biology) YouTube How To Measure Ph Scale For example, a ph of 3 is ten times more acidic than a. Apply the same strategy for representing other types of. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in. A value less than 7 indicates that. Ph. How To Measure Ph Scale.
From www.alamy.com
vector ph scale of acidic,neutral and alkaline value chart for acid and How To Measure Ph Scale The formula for ph is. Ph represents the concentration of hydrogen ions (h+) in a solution and is. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. The range of ph goes from 0 to 14. Ph is measured on a logarithmic scale. A value less. How To Measure Ph Scale.
From mavink.com
Ph Scale Acids And Bases How To Measure Ph Scale The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Apply the same strategy for representing other types of. In this article, we explore the diverse methods and techniques available for ph measurement. A value less than 7 indicates that. The range of ph goes from 0. How To Measure Ph Scale.
From www.alamy.com
The ph scale diagram Stock Vector Image & Art Alamy How To Measure Ph Scale Tell the origin and the logic of using the ph scale. For example, a ph of 3 is ten times more acidic than a. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in. The formula for ph is. There. How To Measure Ph Scale.
From www.dreamstime.com
PH scale diagram stock vector. Illustration of scale 248800856 How To Measure Ph Scale In this article, we explore the diverse methods and techniques available for ph measurement. Ph represents the concentration of hydrogen ions (h+) in a solution and is. The formula for ph is. A value less than 7 indicates that. There are multiple methods of measuring ph. To define the ph scale as a measure of acidity of a solution; Since. How To Measure Ph Scale.
From www.science-sparks.com
What is the pH Scale? How To Measure Ph Scale Ph is measured on a logarithmic scale. Tell the origin and the logic of using the ph scale. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. A value less than 7 indicates that. There are multiple methods of measuring ph. The ph scale measures how strongly acidic. How To Measure Ph Scale.
From www.vectorstock.com
Ph scale diagram Royalty Free Vector Image VectorStock How To Measure Ph Scale Tell the origin and the logic of using the ph scale. The formula for ph is. A value less than 7 indicates that. Ph is measured on a logarithmic scale. When pure water is dropped into a solution of universal indicator, the. The range of ph goes from 0 to 14. The ph scale is logarithmic, meaning that an increase. How To Measure Ph Scale.
From www.bartleby.com
What Does the pH Scale Measure? Free Expert Q&A bartleby How To Measure Ph Scale Tell the origin and the logic of using the ph scale. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Apply the same strategy for. How To Measure Ph Scale.
From www.dreamstime.com
Ph Scale Value Meter or Diet Acids Measure Chart Stock Illustration How To Measure Ph Scale One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in. A value less than 7 indicates that. In this article, we explore the diverse methods and techniques available for ph measurement. Apply the same strategy for representing other types of.. How To Measure Ph Scale.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG How To Measure Ph Scale The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. Ph is measured on a logarithmic scale. Tell the origin and the logic of using the ph scale. The range of ph goes from 0 to 14. In this article, we explore the diverse methods and techniques available for. How To Measure Ph Scale.
From www.alamy.com
Ph scale hires stock photography and images Alamy How To Measure Ph Scale Ph is measured on a logarithmic scale. There are multiple methods of measuring ph. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. The formula for ph is. When pure water is dropped into a solution of universal indicator, the. The range of ph goes from 0 to. How To Measure Ph Scale.
From www.linkedin.com
What is pH Scale? How to Measure pH Value Through pH Scale? How To Measure Ph Scale Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. Ph is measured on a logarithmic scale. A value less than 7 indicates that. The formula for ph is. For example, a ph of 3 is ten times more acidic than a. To define the ph scale as a measure. How To Measure Ph Scale.
From www.dreamstime.com
PH scale diagram stock vector. Illustration of measurement 248488666 How To Measure Ph Scale Ph represents the concentration of hydrogen ions (h+) in a solution and is. Apply the same strategy for representing other types of. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. There are multiple methods of measuring ph. The ph scale is logarithmic, meaning that an. How To Measure Ph Scale.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo How To Measure Ph Scale Ph represents the concentration of hydrogen ions (h+) in a solution and is. To define the ph scale as a measure of acidity of a solution; In this article, we explore the diverse methods and techniques available for ph measurement. For example, a ph of 3 is ten times more acidic than a. When pure water is dropped into a. How To Measure Ph Scale.
From www.expii.com
What Is pH? — Definition & Overview Expii How To Measure Ph Scale In this article, we explore the diverse methods and techniques available for ph measurement. A value less than 7 indicates that. For example, a ph of 3 is ten times more acidic than a. There are multiple methods of measuring ph. Ph represents the concentration of hydrogen ions (h+) in a solution and is. To define the ph scale as. How To Measure Ph Scale.
From www.youtube.com
Test your Body pH Level using the pH Test Strip Acid Alkaline Diet How To Measure Ph Scale Ph represents the concentration of hydrogen ions (h+) in a solution and is. Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. Tell the origin and the logic of using the ph scale. There are multiple methods of measuring ph. For example, a ph of 3 is ten times. How To Measure Ph Scale.
From www.dreamstime.com
The Ph Scale Universal Indicator Ph Color Chart Diagram. Vector How To Measure Ph Scale For example, a ph of 3 is ten times more acidic than a. Apply the same strategy for representing other types of. The range of ph goes from 0 to 14. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. A value less than 7 indicates. How To Measure Ph Scale.
From www.dreamstime.com
PH Scale Indicator Chart Diagram Acidic Alkaline Measure. PH Analysis How To Measure Ph Scale In this article, we explore the diverse methods and techniques available for ph measurement. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in. Apply the same strategy for representing other types of. Tell the origin and the logic of. How To Measure Ph Scale.
From surveylabel.blogspot.com
39 ph scale with labels How To Measure Ph Scale Tell the origin and the logic of using the ph scale. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. In this article, we explore the diverse methods and techniques available for ph measurement. Ph represents the concentration of hydrogen ions (h+) in a solution and is. Apply. How To Measure Ph Scale.
From blog.havells.com
Right pH Level in Drinking Water How Essential? Havells India Blog How To Measure Ph Scale Ph represents the concentration of hydrogen ions (h+) in a solution and is. There are multiple methods of measuring ph. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph. How To Measure Ph Scale.
From www.inchcalculator.com
pH Calculator Inch Calculator How To Measure Ph Scale Ph represents the concentration of hydrogen ions (h+) in a solution and is. Tell the origin and the logic of using the ph scale. Ph is measured on a logarithmic scale. Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. In this article, we explore the diverse methods and. How To Measure Ph Scale.
From www.savemyexams.com
The pH Scale SL IB Chemistry Revision Notes 2025 Save My Exams How To Measure Ph Scale Tell the origin and the logic of using the ph scale. Ph is measured on a logarithmic scale. A value less than 7 indicates that. Apply the same strategy for representing other types of. Since many reactions in everyday life are affected by ph, it's useful to know how to calculate and measure it. The ph scale measures how strongly. How To Measure Ph Scale.
From www.alamy.com
pH scale indicator chart diagram acidic alkaline measure. pH analysis How To Measure Ph Scale The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. In this article, we explore the diverse methods and techniques available for ph measurement. When pure water is dropped into a solution of universal indicator, the. Ph represents the concentration of hydrogen ions (h+) in a solution and is.. How To Measure Ph Scale.
From quizzfullcraig.z19.web.core.windows.net
Explaining The Ph Scale How To Measure Ph Scale The ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. Tell the origin and the logic of using the ph scale. One qualitative measure of the. How To Measure Ph Scale.