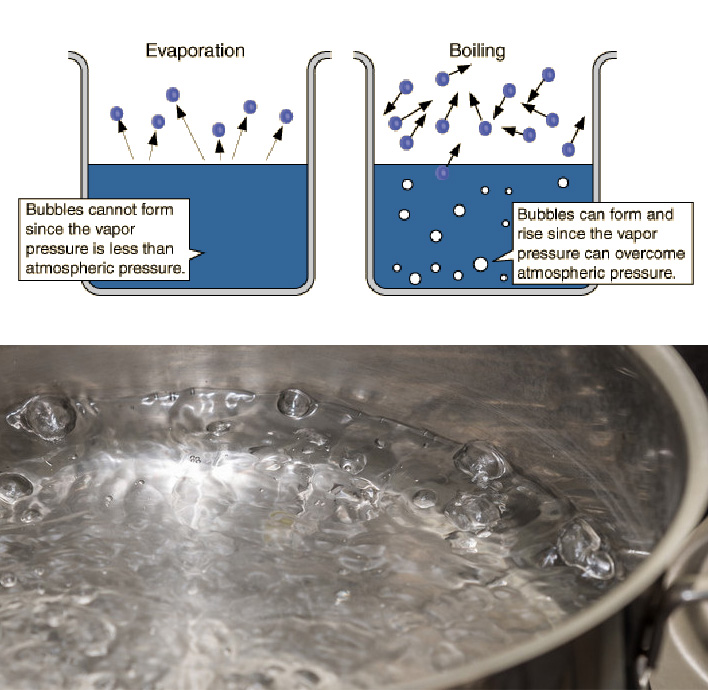
Water Evaporate In A Vacuum . However, the role of fluid dynamics has not been explored sufficiently. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Other than pressure and temperature, the placement of the heater, source and substrate are important factors. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Evaporation is normally done in the ballistic regime (kn > 1). The boiling point of a substance is the temperature at which the. As water evaporates, you no. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units.
from www.watercubedesign.it
As water evaporates, you no. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Other than pressure and temperature, the placement of the heater, source and substrate are important factors. Evaporation is normally done in the ballistic regime (kn > 1). However, the role of fluid dynamics has not been explored sufficiently. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. The boiling point of a substance is the temperature at which the.
Evaporation Watercube Design
Water Evaporate In A Vacuum Other than pressure and temperature, the placement of the heater, source and substrate are important factors. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. However, the role of fluid dynamics has not been explored sufficiently. Other than pressure and temperature, the placement of the heater, source and substrate are important factors. The boiling point of a substance is the temperature at which the. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. As water evaporates, you no. Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Evaporation is normally done in the ballistic regime (kn > 1). Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere.
From saltworkconsultants.com
Physics of evaporation Water Evaporate In A Vacuum Other than pressure and temperature, the placement of the heater, source and substrate are important factors. However, the role of fluid dynamics has not been explored sufficiently. As water evaporates, you no. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Online calculator, figures and tables giving the boiling temperatures of. Water Evaporate In A Vacuum.
From www.labconco.com
RapidVap Vacuum Evaporation Systems Labconco Water Evaporate In A Vacuum Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. As water evaporates, you no. Yes, but in practice,. Water Evaporate In A Vacuum.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Water Evaporate In A Vacuum Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. However, the role of fluid dynamics has not been explored sufficiently. As water evaporates, you no. Online calculator, figures and tables giving the boiling. Water Evaporate In A Vacuum.
From aquadest.cz
Vacuum evaporation Aquadest Water Evaporate In A Vacuum Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Evaporation is normally done in the ballistic regime (kn > 1). Online calculator, figures and tables giving the boiling temperatures of water. Water Evaporate In A Vacuum.
From stock.adobe.com
Rotary vacuum evaporator in chemical laboratory. Close up of glass bulb Water Evaporate In A Vacuum Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Evaporation is normally done in the ballistic regime (kn > 1). Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at. Water Evaporate In A Vacuum.
From www.watercubedesign.it
Evaporation Watercube Design Water Evaporate In A Vacuum Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. The boiling point of a substance is the temperature at which the. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. Evaporation is normally done in the ballistic regime (kn > 1). When you heat. Water Evaporate In A Vacuum.
From www.bbc.co.uk
Evaporation BBC Bitesize Water Evaporate In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Other than pressure and temperature, the placement of the heater, source and substrate are important factors. The boiling point of a substance is the temperature at which. Water Evaporate In A Vacuum.
From sarvowater.com
Mechanical Vapor Sarvo Water Water Evaporate In A Vacuum Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. Evaporation is normally done in the ballistic regime (kn > 1). Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. When you heat a liquid more molecules will leave the liquid into the. Water Evaporate In A Vacuum.
From www.slideserve.com
PPT Separating Mixtures PowerPoint Presentation, free download ID Water Evaporate In A Vacuum However, the role of fluid dynamics has not been explored sufficiently. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Yes, but in practice, it depends on the volume of water, the container, and the performance. Water Evaporate In A Vacuum.
From www.semanticscholar.org
Design, construction and evaluation of a vacuum evaporation system for Water Evaporate In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Evaporation is normally done in the ballistic regime (kn > 1). The boiling point of a substance is the temperature at which the. Water evaporates when the. Water Evaporate In A Vacuum.
From netsolwater.com
What are vacuum evaporation and it uses in treatment of wastewater Water Evaporate In A Vacuum As water evaporates, you no. Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. However, the role of fluid dynamics has not been explored sufficiently. The boiling point of a substance is the temperature at which the. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low. Water Evaporate In A Vacuum.
From mavink.com
Water Vapour Pressure Chart Water Evaporate In A Vacuum Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. Other than pressure and temperature, the placement of the heater, source and substrate are important factors.. Water Evaporate In A Vacuum.
From www.slideserve.com
PPT The Water Cycle and Plants PowerPoint Presentation, free download Water Evaporate In A Vacuum However, the role of fluid dynamics has not been explored sufficiently. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. Water Evaporate In A Vacuum.
From www.pf10.it
EVAPORATORS AND CONCENTRATION UNITS PF10 Water Evaporate In A Vacuum Evaporation is normally done in the ballistic regime (kn > 1). Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. When you heat a liquid more molecules will leave the liquid. Water Evaporate In A Vacuum.
From www.youtube.com
water evaporates in vacuum YouTube Water Evaporate In A Vacuum Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Evaporation is normally done in the ballistic regime (kn > 1). Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Other. Water Evaporate In A Vacuum.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Water Evaporate In A Vacuum However, the role of fluid dynamics has not been explored sufficiently. The boiling point of a substance is the temperature at which the. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Evaporation is normally done. Water Evaporate In A Vacuum.
From melscience.com
Vacuum kettle MEL Physics Water Evaporate In A Vacuum Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. However, the role of fluid dynamics has not been explored sufficiently. Online calculator, figures and tables giving the boiling temperatures of water. Water Evaporate In A Vacuum.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry Water Evaporate In A Vacuum Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. However, the role of fluid dynamics has not been explored sufficiently. Evaporation is normally done in. Water Evaporate In A Vacuum.
From www.semanticscholar.org
Design, construction and evaluation of a vacuum evaporation system for Water Evaporate In A Vacuum Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. Evaporation is normally done in the ballistic regime (kn > 1). As water evaporates, you no. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid. Water Evaporate In A Vacuum.
From youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Water Evaporate In A Vacuum Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. As water evaporates, you no. Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at. Water Evaporate In A Vacuum.
From slideplayer.com
Liquids Chapter ppt download Water Evaporate In A Vacuum As water evaporates, you no. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. The boiling point of a substance is the temperature at which the. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. However, the role of fluid dynamics has not been. Water Evaporate In A Vacuum.
From sciencing.com
Fast Ways to Make Water Evaporate Sciencing Water Evaporate In A Vacuum Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. When you heat a. Water Evaporate In A Vacuum.
From demonstrations.wolfram.com
Adiabatic Evaporation of Water into Vacuum Wolfram Demonstrations Project Water Evaporate In A Vacuum As water evaporates, you no. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Other than pressure and temperature, the placement of the heater, source and substrate are important factors. However, the role of fluid dynamics has not been explored sufficiently. Evaporation is normally done in the ballistic regime (kn >. Water Evaporate In A Vacuum.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Water Evaporate In A Vacuum Evaporation is normally done in the ballistic regime (kn > 1). Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. The boiling point of a substance is the temperature at which the. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules. Water Evaporate In A Vacuum.
From www.pinterpandai.com
Penjelasan Evaporasi atau Penguapan dan Contohnya Water Evaporate In A Vacuum Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. When you heat a liquid more molecules will. Water Evaporate In A Vacuum.
From www.youtube.com
how to use the rotary evaporator for distillation YouTube Water Evaporate In A Vacuum Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. However, the role of fluid dynamics has not been. Water Evaporate In A Vacuum.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Water Evaporate In A Vacuum The boiling point of a substance is the temperature at which the. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. Evaporation is normally done in the ballistic regime (kn > 1). Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Recent experimental thermocouple. Water Evaporate In A Vacuum.
From netsolwater.com
How is water separated from oil using vacuum evaporation Water Evaporate In A Vacuum As water evaporates, you no. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Evaporation is normally done. Water Evaporate In A Vacuum.
From www.aplustopper.com
How can we Separate a Mixture of a Solid and a Liquid using Evaporation Water Evaporate In A Vacuum The boiling point of a substance is the temperature at which the. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Other than. Water Evaporate In A Vacuum.
From slideplayer.com
Intermolecular Forces (IMF). Part II ppt download Water Evaporate In A Vacuum Yes, but in practice, it depends on the volume of water, the container, and the performance of your vacuum pump. Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Evaporation is normally done in the ballistic regime (kn > 1). Recent experimental thermocouple measurements of the temperature field near the interface. Water Evaporate In A Vacuum.
From alchetron.com
Vacuum evaporation Alchetron, The Free Social Encyclopedia Water Evaporate In A Vacuum As water evaporates, you no. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. The boiling point of a substance is the temperature at which the. However, the role of fluid dynamics has not been explored sufficiently. Evaporation is normally done in the ballistic regime (kn > 1). Recent experimental thermocouple. Water Evaporate In A Vacuum.
From young6science3.weebly.com
5Th grade science The Water Cycle Water Evaporate In A Vacuum Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. However, the role of fluid dynamics has not been explored sufficiently. Recent experimental thermocouple measurements of the temperature field near the interface of evaporating water into its vapor have begun to show the role of heat transfer in evaporation. Online calculator, figures and tables. Water Evaporate In A Vacuum.
From kids.britannica.com
evaporation and condensation Kids Britannica Kids Homework Help Water Evaporate In A Vacuum Evaporation is normally done in the ballistic regime (kn > 1). Because evaporation at low pressure conditions (i.e., vacuum conditions) occurs at low temperatures, it takes more energy to. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. The boiling point of a substance is the temperature at which the. When you heat. Water Evaporate In A Vacuum.
From www.reddit.com
ELI5 why does water evaporate even when it's not at its boiling point Water Evaporate In A Vacuum However, the role of fluid dynamics has not been explored sufficiently. Water evaporates when the vapor pressure is greater than partial pressure of water in the atmosphere. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no.. Water Evaporate In A Vacuum.
From www.pinterest.com
Morus Zero is the first vacuum clothes dryer in the world which can Water Evaporate In A Vacuum Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. However, the role of fluid dynamics has not been explored sufficiently. The boiling point of a substance is the temperature at which the. Other than pressure and temperature, the placement of the heater, source and substrate are important factors. When you heat. Water Evaporate In A Vacuum.