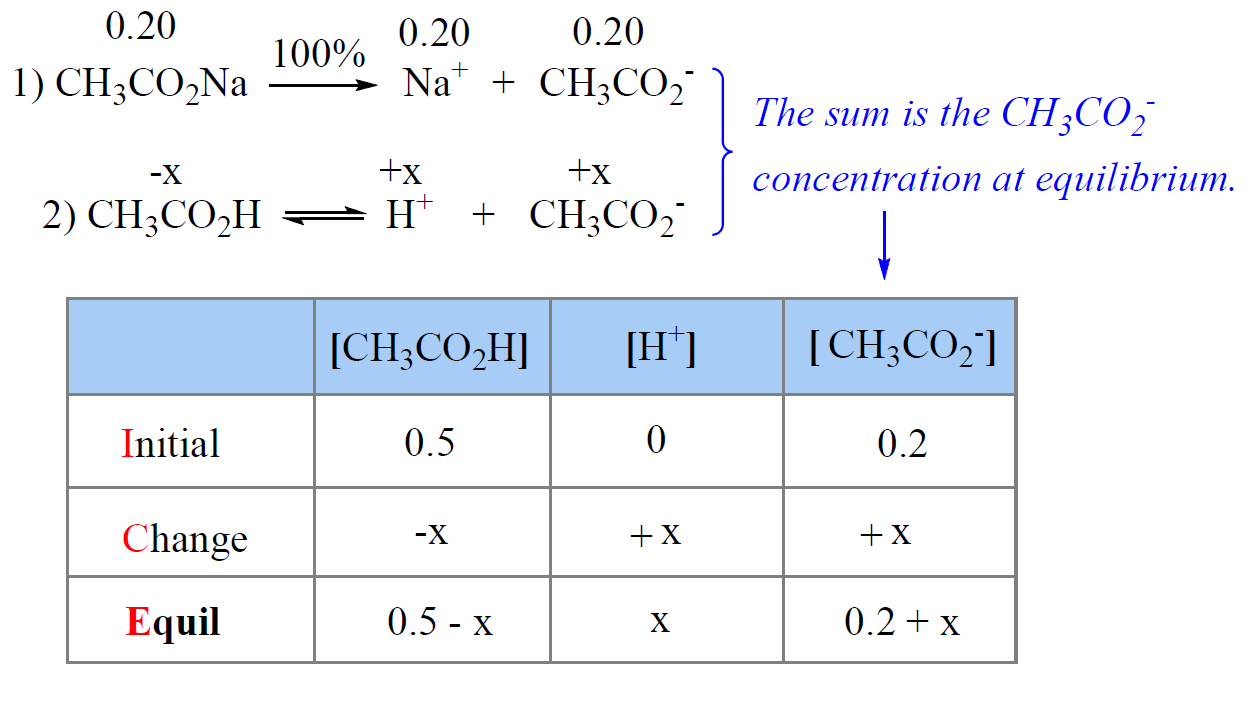
Chemistry Buffer Equation . Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. When you add an acid to a buffer, the conjugate base present in the buffer. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. buffers function through a process of chemical equilibrium. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. It is able to neutralize. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it.
from general.chemistrysteps.com
A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. It is able to neutralize. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. When you add an acid to a buffer, the conjugate base present in the buffer. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffers function through a process of chemical equilibrium.
pH of a Buffer Solution Chemistry Steps
Chemistry Buffer Equation chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. It is able to neutralize. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. buffers function through a process of chemical equilibrium. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. When you add an acid to a buffer, the conjugate base present in the buffer. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Chemistry Buffer Equation this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. When you add an acid to a buffer, the conjugate base present in the buffer. a buffer is a solution that can resist ph change upon the addition of an acidic. Chemistry Buffer Equation.
From www.numerade.com
SOLVED A buffer solution is made by dissolving HC2H3O2 and NaC2H3O2 in Chemistry Buffer Equation a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. Chemistry Buffer Equation.
From www.youtube.com
AP Chem U8 Buffer Calculations Using HendersonHasselbalch Equation Chemistry Buffer Equation chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. When you add an acid to a buffer, the conjugate base present in the. Chemistry Buffer Equation.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Chemistry Buffer Equation It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and. Chemistry Buffer Equation.
From www.youtube.com
Intro to Buffers and the HendersonHasselbalch Equation YouTube Chemistry Buffer Equation A buffer solution is one in which the ph of the solution is resistant to small additions of either a. It is able to neutralize. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. Buffers can react with both strong acids. Chemistry Buffer Equation.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Chemistry Buffer Equation chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. It is able to neutralize. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. this equation relates the ph, the k a of a weak. Chemistry Buffer Equation.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Chemistry Buffer Equation A buffer solution is one in which the ph of the solution is resistant to small additions of either a. It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an. Chemistry Buffer Equation.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Chemistry Buffer Equation chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. When you add. Chemistry Buffer Equation.
From courses.lumenlearning.com
Buffers Chemistry for Majors Chemistry Buffer Equation A buffer solution is one in which the ph of the solution is resistant to small additions of either a. buffers function through a process of chemical equilibrium. It is able to neutralize. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. When you add. Chemistry Buffer Equation.
From study.com
Identifying Buffer Capacity Chemistry Chemistry Buffer Equation buffers function through a process of chemical equilibrium. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. a buffer is a solution that can. Chemistry Buffer Equation.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Chemistry Buffer Equation a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. When you add an acid to a buffer, the conjugate base present in the buffer. It is able to neutralize. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a. Chemistry Buffer Equation.
From www.youtube.com
Buffer Solutions YouTube Chemistry Buffer Equation a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a. Chemistry Buffer Equation.
From classnotes.org.in
Calculation of pH of a Buffer Mixture Chemistry, Class 11, Ionic Chemistry Buffer Equation A buffer solution is one in which the ph of the solution is resistant to small additions of either a. When you add an acid to a buffer, the conjugate base present in the buffer. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is. Chemistry Buffer Equation.
From criticalthinking.cloud
how to solve buffer problems chemistry Chemistry Buffer Equation A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able. Chemistry Buffer Equation.
From www.studypage.in
Buffer Solutions Study Page Chemistry Buffer Equation this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. It is able to neutralize. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. chemical education digital. Chemistry Buffer Equation.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Chemistry Buffer Equation When you add an acid to a buffer, the conjugate base present in the buffer. buffers function through a process of chemical equilibrium. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize. Buffers can react with both strong acids (top) and strong bases. Chemistry Buffer Equation.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Chemistry Buffer Equation When you add an acid to a buffer, the conjugate base present in the buffer. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to. Chemistry Buffer Equation.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Chemistry Buffer Equation buffers function through a process of chemical equilibrium. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize. A buffer solution is one in which the ph of the solution. Chemistry Buffer Equation.
From www.youtube.com
8 Buffer Calculations 3 YouTube Chemistry Buffer Equation chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. buffers function through a process of chemical equilibrium. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution.. Chemistry Buffer Equation.
From www.youtube.com
Intro to Buffer Solutions Henderson Hasselbalch Equation and Acid Base Chemistry Buffer Equation this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffers can react with both strong acids (top) and. Chemistry Buffer Equation.
From www.youtube.com
Acids and Bases Buffer Calculation Past Paper Exam Question Chemistry Buffer Equation A buffer solution is one in which the ph of the solution is resistant to small additions of either a. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize. chemical education digital library (chemed dl) let us now consider the. Chemistry Buffer Equation.
From education-portal.com
AcidBase Buffers Calculating the pH of a Buffered Solution Video Chemistry Buffer Equation a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. It is able to neutralize. buffers function through a process of chemical equilibrium. Buffers can react with both strong. Chemistry Buffer Equation.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Chemistry Buffer Equation chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. A buffer solution is one which resists changes in ph when small quantities of an acid or. Chemistry Buffer Equation.
From www.youtube.com
Buffer capacity Acids and bases AP Chemistry Khan Academy YouTube Chemistry Buffer Equation When you add an acid to a buffer, the conjugate base present in the buffer. buffers function through a process of chemical equilibrium. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. Buffers can react with both strong acids (top). Chemistry Buffer Equation.
From www.osmosis.org
Buffering and HendersonHasselbalch equation Video Osmosis Chemistry Buffer Equation chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. buffers function through a process of chemical equilibrium. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution.. Chemistry Buffer Equation.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Chemistry Buffer Equation A buffer solution is one in which the ph of the solution is resistant to small additions of either a. When you add an acid to a buffer, the conjugate base present in the buffer. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. chemical education digital library. Chemistry Buffer Equation.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Chemistry Buffer Equation A buffer solution is one in which the ph of the solution is resistant to small additions of either a. this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. a buffer is a solution that can resist ph change upon. Chemistry Buffer Equation.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Chemistry Buffer Equation A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. a buffer is a solution that can resist ph change upon. Chemistry Buffer Equation.
From classnotes.org.in
Calculation of pH of a Buffer Mixture Chemistry, Class 11, Ionic Chemistry Buffer Equation A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. this equation. Chemistry Buffer Equation.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Chemistry Buffer Equation this equation relates the ph, the k a of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers can react with both strong acids (top) and strong bases (bottom). Chemistry Buffer Equation.
From www.youtube.com
Handerson Hasselbalch Equation Buffer Equation Acid Base And Chemistry Buffer Equation It is able to neutralize. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. A buffer solution is one in which the ph of the solution is resistant to small additions of either a. Buffers can react with both strong acids (top) and strong bases (bottom). Chemistry Buffer Equation.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Chemistry Buffer Equation It is able to neutralize. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers can react with both strong acids (top) and strong bases (bottom). Chemistry Buffer Equation.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Chemistry Buffer Equation A buffer solution is one in which the ph of the solution is resistant to small additions of either a. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. It is able to neutralize. this equation relates the ph, the k a of a weak. Chemistry Buffer Equation.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Chemistry Buffer Equation Buffers can react with both strong acids (top) and strong bases (bottom) to minimize. chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which. When you add an acid to a buffer, the conjugate base present in the buffer. A buffer solution is one in which the. Chemistry Buffer Equation.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps Chemistry Buffer Equation buffers function through a process of chemical equilibrium. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. When you add an acid to a buffer, the conjugate base present in the buffer. It is able to neutralize. chemical education digital library (chemed dl) let. Chemistry Buffer Equation.