Acid Definition Quizlet . The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. A base is a molecule or ion able to accept a. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three rules to remember. A substance that decreases the. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. The process of mixing acids and bases to form a neutral solution. Any compound that increases the number of hydrogen (hydronium ions) when dissolved in water. (a) vinegar comes in a variety of types, but all contain acetic acid. The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. While these definitions don't contradict each other, they do vary in how inclusive they are. Study with quizlet and memorize flashcards containing terms like acids, bases,. There are several methods of defining acids and bases.
from www.worksheetsplanet.com
(a) vinegar comes in a variety of types, but all contain acetic acid. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. While these definitions don't contradict each other, they do vary in how inclusive they are. The earliest definition of acids and bases is arrhenius's definition which states that: The process of mixing acids and bases to form a neutral solution. Any compound that increases the number of hydrogen (hydronium ions) when dissolved in water. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three rules to remember. A base is a molecule or ion able to accept a. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. There are several methods of defining acids and bases.
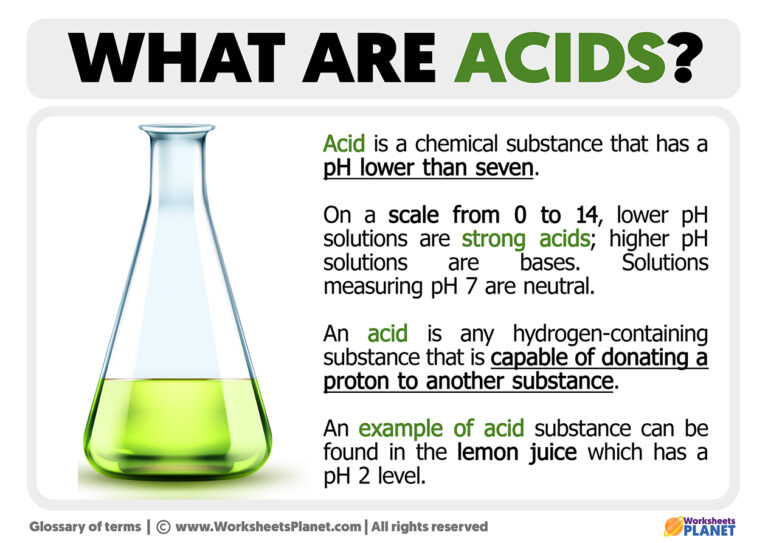
What is an Acid Definition of Acid
Acid Definition Quizlet Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three rules to remember. The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. (a) vinegar comes in a variety of types, but all contain acetic acid. Study with quizlet and memorize flashcards containing terms like acids, bases,. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three rules to remember. An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. While these definitions don't contradict each other, they do vary in how inclusive they are. A substance that decreases the. The process of mixing acids and bases to form a neutral solution. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. Any compound that increases the number of hydrogen (hydronium ions) when dissolved in water. A base is a molecule or ion able to accept a. There are several methods of defining acids and bases. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water.
From www.slideserve.com
PPT Acids, pH, and Buffers Some Basic Chemistry for Biological Science PowerPoint Acid Definition Quizlet The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three rules to remember. An acid is a chemical species that donates. Acid Definition Quizlet.
From quizlet.com
Fatty Acid Structure Diagram Quizlet Acid Definition Quizlet Study with quizlet and memorize flashcards containing terms like acids, bases,. Any compound that increases the number of hydrogen (hydronium ions) when dissolved in water. (a) vinegar comes in a variety of types, but all contain acetic acid. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. While these. Acid Definition Quizlet.
From quizlet.com
20 Common Amino Acids (w/ All the Info) Diagram Quizlet Acid Definition Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: The process of mixing acids and bases to form a neutral solution. While these definitions don't contradict each other, they do vary in how inclusive they are. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water.. Acid Definition Quizlet.
From www.youtube.com
8th class Science Ch 07 Acids define acids Properties of Acids Sba assessment base Acid Definition Quizlet The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. A substance that decreases the. (a) vinegar comes in a variety of types, but all contain acetic acid. An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. Any compound that increases. Acid Definition Quizlet.
From mmerevise.co.uk
Acids and Bases Questions and Revision MME Acid Definition Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. A substance. Acid Definition Quizlet.
From www.youtube.com
Acids (Definition, physical properties and chemical properties) YouTube Acid Definition Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: Study with quizlet and memorize flashcards containing terms like acids, bases,. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three rules to remember. The process of mixing acids and bases to form a neutral solution. An acid. Acid Definition Quizlet.
From quizlet.com
CC8 Acids and alkalis FT Diagram Quizlet Acid Definition Quizlet Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. There are several methods of defining acids and bases. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. Study. Acid Definition Quizlet.
From www.blendspace.com
Acids And Bases Introduction Lessons Blendspace Acid Definition Quizlet While these definitions don't contradict each other, they do vary in how inclusive they are. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Any compound that increases the number of hydrogen (hydronium ions) when dissolved in water. There are several methods of defining acids and bases. The process. Acid Definition Quizlet.
From www.youtube.com
acid define acid acid definition chemistry acid definition in english YouTube Acid Definition Quizlet A base is a molecule or ion able to accept a. The earliest definition of acids and bases is arrhenius's definition which states that: The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. There are several methods of defining acids and bases. Study with quizlet and. Acid Definition Quizlet.
From www.worksheetsplanet.com
What is an Acid Definition of Acid Acid Definition Quizlet The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. There are several methods of defining acids and bases. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved. Acid Definition Quizlet.
From hxewyxqxm.blob.core.windows.net
Quizlet Saturated Fatty Acids And Unsaturated Fatty Acids Differ In at Steven Donaldson blog Acid Definition Quizlet The process of mixing acids and bases to form a neutral solution. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Study with quizlet and memorize flashcards containing terms like acids, bases,. A base is a molecule or ion able to accept a. Study with quizlet and memorize flashcards. Acid Definition Quizlet.
From quizlet.com
Amino Acid The molecular tool box Diagram Quizlet Acid Definition Quizlet The process of mixing acids and bases to form a neutral solution. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the. Acid Definition Quizlet.
From studylib.net
Acid Base Review Answer the questions below. 1. Define Acid Acid Definition Quizlet Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three rules to remember. A base is a molecule or ion able to accept a. A substance that decreases the. The process of mixing acids and bases to form a neutral solution. There are several methods of defining acids and bases. The. Acid Definition Quizlet.
From quizlet.com
Amino Acid Groups Diagram Quizlet Acid Definition Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. The process of mixing acids and bases to form. Acid Definition Quizlet.
From quizlet.com
acid fast Diagram Quizlet Acid Definition Quizlet The process of mixing acids and bases to form a neutral solution. The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. A base is a molecule or ion able to accept a. There are several methods of defining acids and bases. (a) vinegar comes in a. Acid Definition Quizlet.
From www.slideserve.com
PPT Acid Definitions PowerPoint Presentation, free download ID6398687 Acid Definition Quizlet A substance that decreases the. Study with quizlet and memorize flashcards containing terms like acids, bases,. Any compound that increases the number of hydrogen (hydronium ions) when dissolved in water. There are several methods of defining acids and bases. A base is a molecule or ion able to accept a. The higher the concentration of hydrogen ions produced by an. Acid Definition Quizlet.
From studycampuskatie.z5.web.core.windows.net
Bronsted Lowry Acids And Bases Worksheets Key Acid Definition Quizlet (a) vinegar comes in a variety of types, but all contain acetic acid. While these definitions don't contradict each other, they do vary in how inclusive they are. The process of mixing acids and bases to form a neutral solution. The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a chemical species that. Acid Definition Quizlet.
From quizlet.com
Acid pKa Diagram Quizlet Acid Definition Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: While these definitions don't contradict each other, they do vary in how inclusive they are. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. The higher the concentration of hydrogen ions produced by an acid, the. Acid Definition Quizlet.
From quizlet.com
Amino acid non polar Diagram Quizlet Acid Definition Quizlet Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. The process of mixing acids and bases to form a neutral solution. While these definitions don't contradict each other, they do vary in how inclusive they are. The higher the concentration of hydrogen ions produced by an acid, the higher. Acid Definition Quizlet.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Acid Definition Quizlet The process of mixing acids and bases to form a neutral solution. (a) vinegar comes in a variety of types, but all contain acetic acid. A substance that decreases the. While these definitions don't contradict each other, they do vary in how inclusive they are. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?,. Acid Definition Quizlet.
From quizlet.com
BIO 475 BioChem Amino Acids 2022 Diagram Quizlet Acid Definition Quizlet The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. A substance that decreases the. An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. While these definitions don't contradict each other, they do vary. Acid Definition Quizlet.
From study.com
Acid & Base Anhydrides Definition & Examples Video & Lesson Transcript Acid Definition Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. There are. Acid Definition Quizlet.
From quizlet.com
The Amino Acids Diagram Quizlet Acid Definition Quizlet (a) vinegar comes in a variety of types, but all contain acetic acid. The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three rules to remember. Study with. Acid Definition Quizlet.
From sciencenotes.org
Arrhenius Acids and Bases Acid Definition Quizlet A base is a molecule or ion able to accept a. A substance that decreases the. Study with quizlet and memorize flashcards containing terms like acids, bases,. (a) vinegar comes in a variety of types, but all contain acetic acid. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and. Acid Definition Quizlet.
From quizlet.com
Amino acids Diagram Quizlet Acid Definition Quizlet A substance that decreases the. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. While these definitions don't contradict each other, they do vary in how inclusive they are.. Acid Definition Quizlet.
From www.yourdictionary.com
Difference Between Acids and Bases Key Properties YourDictionary Acid Definition Quizlet The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. While these definitions don't contradict each other, they do vary in how inclusive they are. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?,. Acid Definition Quizlet.
From quizlet.com
MCAT Amino Acids Diagram Quizlet Acid Definition Quizlet The process of mixing acids and bases to form a neutral solution. A substance that decreases the. (a) vinegar comes in a variety of types, but all contain acetic acid. The earliest definition of acids and bases is arrhenius's definition which states that: There are several methods of defining acids and bases. A base is a molecule or ion able. Acid Definition Quizlet.
From www.youtube.com
1 Amino Acids General structure, Classification, Significance Amino acid Chemistry1 Acid Definition Quizlet Any compound that increases the number of hydrogen (hydronium ions) when dissolved in water. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. The process of mixing acids and bases to form a neutral solution. The higher the concentration of hydrogen ions produced by an acid, the higher its. Acid Definition Quizlet.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable Education Group Acid Definition Quizlet The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. (a) vinegar comes in a variety of types, but all contain acetic acid. A substance that decreases the. Study with quizlet and memorize flashcards containing terms like acids will begin with what element?, what are the three. Acid Definition Quizlet.
From mcq4all.com
Best Notes of Acids, Bases, and Salts Class 10 MCQ4ALL Acid Definition Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: The process of mixing acids and bases to form a neutral solution. While these definitions don't contradict each other, they do vary in how inclusive they are. An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. (a) vinegar comes in a. Acid Definition Quizlet.
From proper-cooking.info
Functions Of Acids Acid Definition Quizlet An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. There are several methods of defining acids and bases. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. An acid is a substance that forms hydrogen ions h + when dissolved in water, and. Acid Definition Quizlet.
From drcalef.com
Acids Acid Definition Quizlet Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions \(\left( \ce{h^+} \right)\) when dissolved in water. A substance that decreases the. An acid is a substance. Acid Definition Quizlet.
From sci-wani.com
Acid Acid Definition Quizlet The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the ph of the solution. Any compound that increases the number of hydrogen (hydronium ions) when dissolved in water. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions. Acid Definition Quizlet.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 3 PowerPoint Presentation, free download ID9094509 Acid Definition Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. A base is a molecule or ion able to accept a. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and. Acid Definition Quizlet.
From printablefullbeaky.z21.web.core.windows.net
How Do Names Of Binary Acids Begin Acid Definition Quizlet An acid is a chemical species that donates protons or hydrogen ions and/or accepts electrons. The earliest definition of acids and bases is arrhenius's definition which states that: (a) vinegar comes in a variety of types, but all contain acetic acid. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in. Acid Definition Quizlet.