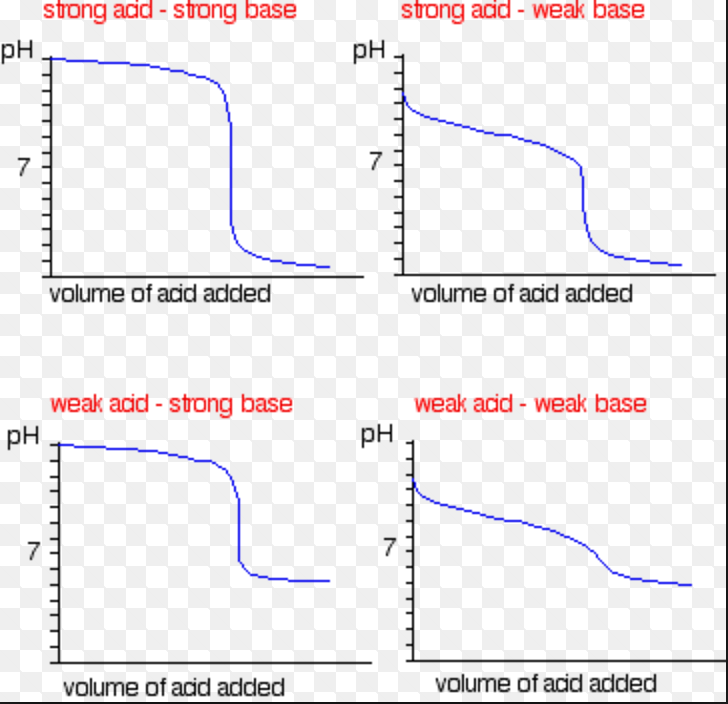
Hypothesis For Titration Curve . It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. Write about the reaction you will be using, including the equation and the conditions required. For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. Calculate initial concentrations of monoprotic acids from titration. In the reaction the acid and base react in a one to one ratio. Include details of the indicator stating the expected color change and writing a brief.
from classnotes.org.in
In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. In the reaction the acid and base react in a one to one ratio. Write about the reaction you will be using, including the equation and the conditions required. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. Calculate initial concentrations of monoprotic acids from titration. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Include details of the indicator stating the expected color change and writing a brief. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration.
Acid Base Titration using Indicator Chemistry, Class 11, Ionic
Hypothesis For Titration Curve In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. Write about the reaction you will be using, including the equation and the conditions required. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Include details of the indicator stating the expected color change and writing a brief. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. In the reaction the acid and base react in a one to one ratio. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. Calculate initial concentrations of monoprotic acids from titration. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Write about the reaction you will be using, including the equation and the conditions required. In this experiment,. Hypothesis For Titration Curve.
From www.purechemistry.org
Precipitation Titration Purechemistry Hypothesis For Titration Curve Include details of the indicator stating the expected color change and writing a brief. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration. Hypothesis For Titration Curve.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Hypothesis For Titration Curve The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Include details of the indicator stating the expected color change and writing a brief. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. It is based on the neutralization reaction, where an acid and. Hypothesis For Titration Curve.
From www.researchgate.net
Potentiometric titration curves and first derivates of native and Hypothesis For Titration Curve Calculate initial concentrations of monoprotic acids from titration. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Write about the reaction you will be using, including the equation and the conditions required. In this experiment, the titration curve for a neutralisation reaction will be drawn. Hypothesis For Titration Curve.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Hypothesis For Titration Curve Identify if an unknown acid is weak or strong and monoprotic or polyprotic. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. The titration of a. Hypothesis For Titration Curve.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Calculate initial concentrations of monoprotic acids from titration. For a titration, the introduction should include information about what you. Hypothesis For Titration Curve.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Hypothesis For Titration Curve In the reaction the acid and base react in a one to one ratio. Include details of the indicator stating the expected color change and writing a brief. Calculate initial concentrations of monoprotic acids from titration. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. For a titration, the introduction should include information about what you. Hypothesis For Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Hypothesis For Titration Curve Write about the reaction you will be using, including the equation and the conditions required. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. Calculate initial concentrations of monoprotic acids from titration. Identify if an unknown acid is weak or strong and monoprotic or. Hypothesis For Titration Curve.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Hypothesis For Titration Curve In the reaction the acid and base react in a one to one ratio. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. For a titration, the. Hypothesis For Titration Curve.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown Hypothesis For Titration Curve Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. For a titration, the introduction should include information about what you hope to find out and what substance or product you will. Hypothesis For Titration Curve.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Hypothesis For Titration Curve Write about the reaction you will be using, including the equation and the conditions required. Include details of the indicator stating the expected color change and writing a brief. In the reaction the acid and base react in a one to one ratio. It is based on the neutralization reaction, where an acid and a base react to form water. Hypothesis For Titration Curve.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Hypothesis For Titration Curve Identify if an unknown acid is weak or strong and monoprotic or polyprotic. For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide. Hypothesis For Titration Curve.
From www.tessshebaylo.com
Karl Fischer Titration Equation Tessshebaylo Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of. Hypothesis For Titration Curve.
From www.chegg.com
Solved Titration curve 14 12 10 6 4 0 0 10 20 30 40 50 60 70 Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. Calculate initial concentrations of monoprotic acids from titration. For a titration,. Hypothesis For Titration Curve.
From www.numerade.com
SOLVED Titrations curve is shown for the titration of a weak base with Hypothesis For Titration Curve Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. In the reaction the acid and base react in a one to one ratio. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. Calculate initial concentrations. Hypothesis For Titration Curve.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Hypothesis For Titration Curve In the reaction the acid and base react in a one to one ratio. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. It is based on the neutralization reaction, where an acid and a base react to form. Hypothesis For Titration Curve.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Hypothesis For Titration Curve Identify if an unknown acid is weak or strong and monoprotic or polyprotic. For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide. Hypothesis For Titration Curve.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Hypothesis For Titration Curve It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. Include details of. Hypothesis For Titration Curve.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Hypothesis For Titration Curve Identify if an unknown acid is weak or strong and monoprotic or polyprotic. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Include details of the indicator stating the expected color change and writing a brief. Write about the reaction you will be using, including the equation and the conditions required. Titration. Hypothesis For Titration Curve.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Hypothesis For Titration Curve Include details of the indicator stating the expected color change and writing a brief. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with. Hypothesis For Titration Curve.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Hypothesis For Titration Curve Include details of the indicator stating the expected color change and writing a brief. In the reaction the acid and base react in a one to one ratio. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. The reaction of the weak acid, acetic. Hypothesis For Titration Curve.
From mungfali.com
Titration Curve Labeled Hypothesis For Titration Curve The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. Include details of the indicator stating the expected color change and writing a brief. Calculate initial concentrations of monoprotic acids from titration. It is based on the neutralization reaction, where. Hypothesis For Titration Curve.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Hypothesis For Titration Curve In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the. Hypothesis For Titration Curve.
From www.researchgate.net
Theoretical titration curves. The calculated curves are shown in red Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted. Hypothesis For Titration Curve.
From www.studocu.com
Titration Curves Interpretation of titration curves strong Acid Hypothesis For Titration Curve In the reaction the acid and base react in a one to one ratio. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Identify if an unknown acid is weak or strong and monoprotic or polyprotic. Write about the reaction you will be using, including the equation and the conditions required. In. Hypothesis For Titration Curve.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Hypothesis For Titration Curve Calculate initial concentrations of monoprotic acids from titration. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titration is the process of determining the concentration. Hypothesis For Titration Curve.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Include. Hypothesis For Titration Curve.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Calculate initial concentrations of monoprotic acids from titration. Identify if an unknown acid is weak or strong and. Hypothesis For Titration Curve.
From www.researchgate.net
Potentiometric titration curve of blank and (0.5g, 1g and 1.5g) of red Hypothesis For Titration Curve Include details of the indicator stating the expected color change and writing a brief. Calculate initial concentrations of monoprotic acids from titration. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. It is based on the neutralization reaction, where an acid and a base react to form water and a salt.. Hypothesis For Titration Curve.
From solvedlib.com
The graph below shows the titration curves for two mo… SolvedLib Hypothesis For Titration Curve For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. It is based on the neutralization reaction, where an acid and a base react to form water and. Hypothesis For Titration Curve.
From www.writework.com
Titration of amino acids WriteWork Hypothesis For Titration Curve The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. In the reaction the acid and base react in a one to one ratio. For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. Include details of the indicator. Hypothesis For Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Hypothesis For Titration Curve In the reaction the acid and base react in a one to one ratio. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. Include details of the indicator stating the expected color change and writing a brief. The reaction of the weak acid, acetic. Hypothesis For Titration Curve.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. Include details of the indicator stating the expected color change and. Hypothesis For Titration Curve.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Hypothesis For Titration Curve The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Calculate initial concentrations of monoprotic acids from titration. Include details of the indicator stating the expected color change and writing a brief. Titration is the process of determining the concentration of an unknown solution using a. Hypothesis For Titration Curve.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Hypothesis For Titration Curve In this experiment, the titration curve for a neutralisation reaction will be drawn and the curve will be interpreted on the basis of ionic theory. For a titration, the introduction should include information about what you hope to find out and what substance or product you will be analyzing. The reaction of the weak acid, acetic acid, with a strong. Hypothesis For Titration Curve.