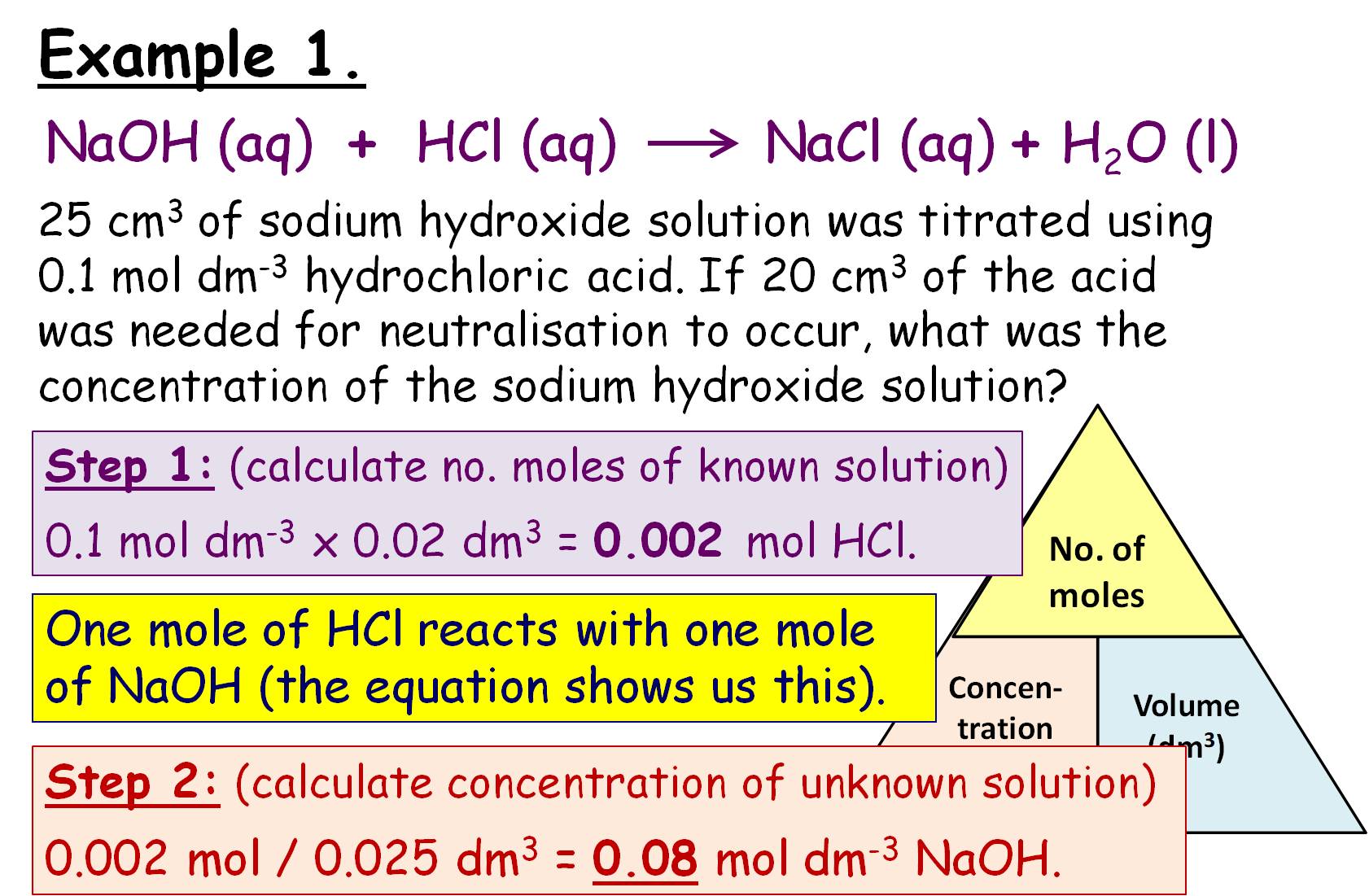
How To Calculate Concentration From Titration Results . Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). This video walks through common examples of calculation problems for titration. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. A titration experiment aims to determine the concentration of an unknown solution by using. It takes 25ml of naoh. The amount of added titrant is determined from its concentration and volume: To determine the concentration of an acid or base using titration, utilize the titration formula: M_a v_a = m_b v_b, where m_a and v_a represent the. The process of calculating concentration from titration data is described and illustrated. How to do titration calculations. When the reaction between the analyte and titrant is. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration calculations are used to find the concentration of unknown solutions. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. They can also be used to calculate the ph after a given point during a titration.
from www.tes.com
This video walks through common examples of calculation problems for titration. The process of calculating concentration from titration data is described and illustrated. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. They can also be used to calculate the ph after a given point during a titration. To determine the concentration of an acid or base using titration, utilize the titration formula: A titration experiment aims to determine the concentration of an unknown solution by using. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. The amount of added titrant is determined from its concentration and volume: M_a v_a = m_b v_b, where m_a and v_a represent the.
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d
How To Calculate Concentration From Titration Results How to do titration calculations. The process of calculating concentration from titration data is described and illustrated. It takes 25ml of naoh. How to do titration calculations. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. When the reaction between the analyte and titrant is. A titration experiment aims to determine the concentration of an unknown solution by using. M_a v_a = m_b v_b, where m_a and v_a represent the. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. To determine the concentration of an acid or base using titration, utilize the titration formula: This video walks through common examples of calculation problems for titration. They can also be used to calculate the ph after a given point during a titration. Titration calculations are used to find the concentration of unknown solutions. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration.
From celtjcvb.blob.core.windows.net
Titration In Simple Terms at John Haslett blog How To Calculate Concentration From Titration Results A titration experiment aims to determine the concentration of an unknown solution by using. They can also be used to calculate the ph after a given point during a titration. Titration calculations are used to find the concentration of unknown solutions. The process of calculating concentration from titration data is described and illustrated. Let's assume you are titrating a strong. How To Calculate Concentration From Titration Results.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Calculate Concentration From Titration Results N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. When the reaction between the analyte and titrant is. A titration experiment aims to determine the concentration of an unknown solution by using. The process of calculating concentration from titration data is described and illustrated.. How To Calculate Concentration From Titration Results.
From joiuugyhn.blob.core.windows.net
Dilution Calculator Concentration at Bernard Butler blog How To Calculate Concentration From Titration Results The amount of added titrant is determined from its concentration and volume: To determine the concentration of an acid or base using titration, utilize the titration formula: A titration experiment aims to determine the concentration of an unknown solution by using. How to do titration calculations. It takes 25ml of naoh. Titration calculations are used to find the concentration of. How To Calculate Concentration From Titration Results.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS How To Calculate Concentration From Titration Results When the reaction between the analyte and titrant is. Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. M_a v_a = m_b v_b, where m_a and v_a represent the. The amount of added titrant is determined from its concentration and volume: It. How To Calculate Concentration From Titration Results.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration From Titration Results The amount of added titrant is determined from its concentration and volume: When the reaction between the analyte and titrant is. To determine the concentration of an acid or base using titration, utilize the titration formula: The process of calculating concentration from titration data is described and illustrated. M_a v_a = m_b v_b, where m_a and v_a represent the. How. How To Calculate Concentration From Titration Results.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog How To Calculate Concentration From Titration Results They can also be used to calculate the ph after a given point during a titration. How to do titration calculations. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration calculations are used to find the concentration of unknown solutions. To determine the concentration of an acid or base using titration, utilize the. How To Calculate Concentration From Titration Results.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube How To Calculate Concentration From Titration Results In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. When the reaction between the analyte and titrant is. The amount of added titrant is determined from its concentration and volume: It takes 25ml of naoh. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. How To Calculate Concentration From Titration Results.
From calculatorshub.net
OH From Your Titrations Calculator Online How To Calculate Concentration From Titration Results How to do titration calculations. They can also be used to calculate the ph after a given point during a titration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). This video walks through common examples of calculation problems for titration. Titration is a method to determine the unknown. How To Calculate Concentration From Titration Results.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog How To Calculate Concentration From Titration Results N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). It takes 25ml of naoh. They can also be used to calculate the ph after. How To Calculate Concentration From Titration Results.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution How To Calculate Concentration From Titration Results It takes 25ml of naoh. Titration calculations are used to find the concentration of unknown solutions. When the reaction between the analyte and titrant is. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. This video walks through common examples of calculation problems for. How To Calculate Concentration From Titration Results.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration How To Calculate Concentration From Titration Results M_a v_a = m_b v_b, where m_a and v_a represent the. It takes 25ml of naoh. To determine the concentration of an acid or base using titration, utilize the titration formula: Titration calculations are used to find the concentration of unknown solutions. When the reaction between the analyte and titrant is. How to do titration calculations. The process of calculating. How To Calculate Concentration From Titration Results.
From mungfali.com
Acid Base Titration Calculation How To Calculate Concentration From Titration Results A titration experiment aims to determine the concentration of an unknown solution by using. The process of calculating concentration from titration data is described and illustrated. When the reaction between the analyte and titrant is. How to do titration calculations. They can also be used to calculate the ph after a given point during a titration. To determine the concentration. How To Calculate Concentration From Titration Results.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog How To Calculate Concentration From Titration Results It takes 25ml of naoh. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). They can also be used to calculate the ph after a given point during a titration. A titration experiment aims to determine the concentration of an unknown solution by using. M_a v_a = m_b v_b,. How To Calculate Concentration From Titration Results.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic How To Calculate Concentration From Titration Results M_a v_a = m_b v_b, where m_a and v_a represent the. To determine the concentration of an acid or base using titration, utilize the titration formula: Titration calculations are used to find the concentration of unknown solutions. This video walks through common examples of calculation problems for titration. When the reaction between the analyte and titrant is. N (mol) =. How To Calculate Concentration From Titration Results.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration From Titration Results Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. A titration experiment aims to determine the concentration of an unknown solution by using. This video walks through common examples of calculation problems for titration. The amount of added titrant is determined from its concentration and volume: M_a v_a. How To Calculate Concentration From Titration Results.
From mungfali.com
Endpoint Titration Curve How To Calculate Concentration From Titration Results N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. The process of calculating concentration from titration data is described and illustrated. The amount of added titrant is determined from its concentration and volume: A titration experiment aims to determine the concentration of an unknown. How To Calculate Concentration From Titration Results.
From exoafzput.blob.core.windows.net
Titration Graph Point at Arleen McKoy blog How To Calculate Concentration From Titration Results To determine the concentration of an acid or base using titration, utilize the titration formula: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. M_a v_a = m_b v_b, where m_a and v_a. How To Calculate Concentration From Titration Results.
From chemistry.stackexchange.com
acid base Does diluting the titration flask change the molarity How To Calculate Concentration From Titration Results A titration experiment aims to determine the concentration of an unknown solution by using. The amount of added titrant is determined from its concentration and volume: Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. N (mol) = c (mol /l) * v (l) and the amount of. How To Calculate Concentration From Titration Results.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Calculate Concentration From Titration Results The process of calculating concentration from titration data is described and illustrated. M_a v_a = m_b v_b, where m_a and v_a represent the. Titration calculations are used to find the concentration of unknown solutions. To determine the concentration of an acid or base using titration, utilize the titration formula: The amount of added titrant is determined from its concentration and. How To Calculate Concentration From Titration Results.
From studyritualizes.z4.web.core.windows.net
How To Find Final Concentration How To Calculate Concentration From Titration Results How to do titration calculations. Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). M_a v_a = m_b v_b, where m_a and. How To Calculate Concentration From Titration Results.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals How To Calculate Concentration From Titration Results Titration calculations are used to find the concentration of unknown solutions. The process of calculating concentration from titration data is described and illustrated. The amount of added titrant is determined from its concentration and volume: To determine the concentration of an acid or base using titration, utilize the titration formula: A titration experiment aims to determine the concentration of an. How To Calculate Concentration From Titration Results.
From www.youtube.com
Titration Calculations YouTube How To Calculate Concentration From Titration Results It takes 25ml of naoh. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Titration calculations are used to find the concentration of. How To Calculate Concentration From Titration Results.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Calculate Concentration From Titration Results To determine the concentration of an acid or base using titration, utilize the titration formula: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. It takes 25ml of naoh. Let's assume you are. How To Calculate Concentration From Titration Results.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Calculate Concentration From Titration Results When the reaction between the analyte and titrant is. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the. A titration experiment aims to. How To Calculate Concentration From Titration Results.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Calculate Concentration From Titration Results How to do titration calculations. Titration calculations are used to find the concentration of unknown solutions. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The amount of added titrant is determined from its concentration and volume: A titration experiment aims to determine the concentration of an unknown solution by using. N (mol) =. How To Calculate Concentration From Titration Results.
From labthink.netlify.app
What is titration and how does it work How To Calculate Concentration From Titration Results In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. This video walks through common examples of calculation problems for titration. To determine the concentration of an acid or base using titration, utilize the titration formula: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. How To Calculate Concentration From Titration Results.
From learningschoolmysticis9.z22.web.core.windows.net
How To Do Titrations In Chemistry How To Calculate Concentration From Titration Results Titration calculations are used to find the concentration of unknown solutions. M_a v_a = m_b v_b, where m_a and v_a represent the. A titration experiment aims to determine the concentration of an unknown solution by using. When the reaction between the analyte and titrant is. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. How To Calculate Concentration From Titration Results.
From ceavsgvd.blob.core.windows.net
How To Calculate Acid Concentration From Titration at Susan Carrier blog How To Calculate Concentration From Titration Results When the reaction between the analyte and titrant is. It takes 25ml of naoh. To determine the concentration of an acid or base using titration, utilize the titration formula: The process of calculating concentration from titration data is described and illustrated. M_a v_a = m_b v_b, where m_a and v_a represent the. This video walks through common examples of calculation. How To Calculate Concentration From Titration Results.
From www.chegg.com
Solved What is the average concentration of your How To Calculate Concentration From Titration Results The process of calculating concentration from titration data is described and illustrated. This video walks through common examples of calculation problems for titration. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. M_a v_a = m_b v_b, where m_a and v_a represent the. In a titration, 25.0 cm. How To Calculate Concentration From Titration Results.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d How To Calculate Concentration From Titration Results This video walks through common examples of calculation problems for titration. They can also be used to calculate the ph after a given point during a titration. M_a v_a = m_b v_b, where m_a and v_a represent the. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and. How To Calculate Concentration From Titration Results.