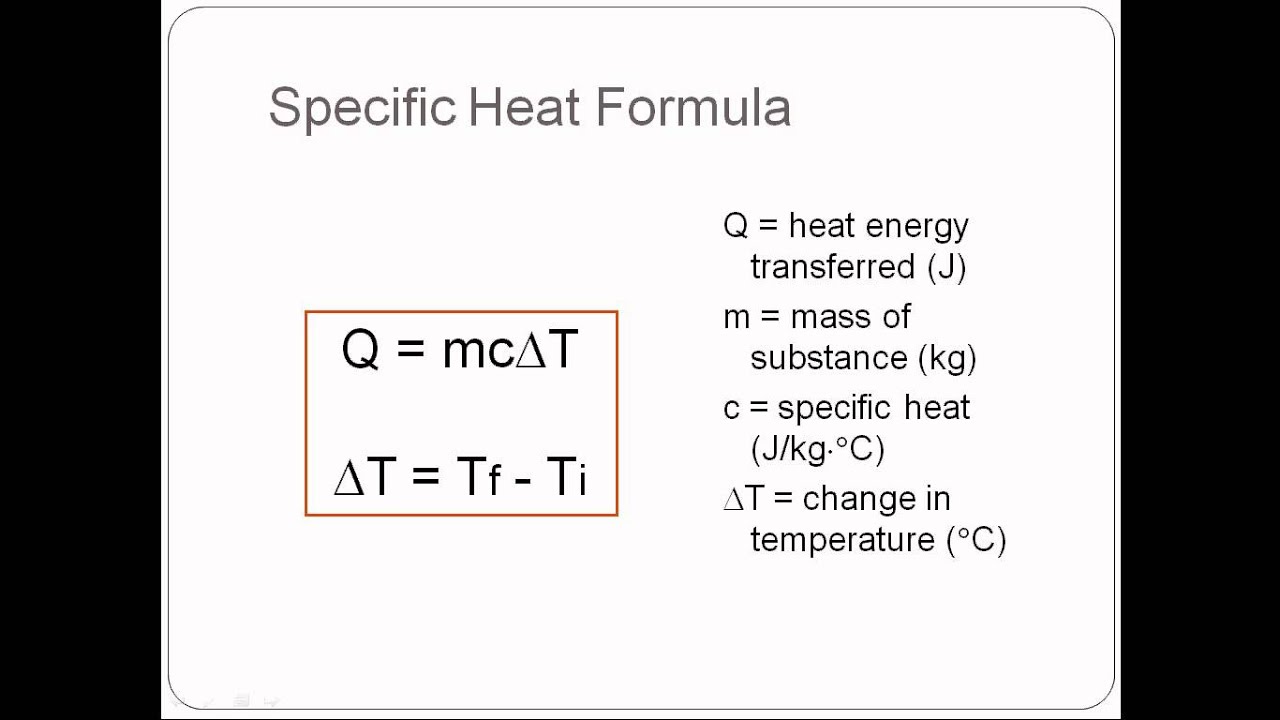
Heat Transfer Q Units . The quantitative relationship between heat transfer and temperature change contains all three factors: Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan,. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. The heat flux can be ˙q determined by. The primary objective of this. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the.
from www.youtube.com
Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The heat flux can be ˙q determined by. The primary objective of this. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The quantitative relationship between heat transfer and temperature change contains all three factors: Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan,. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance.
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi
Heat Transfer Q Units The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan,. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The primary objective of this. The heat flux can be ˙q determined by. The quantitative relationship between heat transfer and temperature change contains all three factors:
From www.slideserve.com
PPT ChE306 Heat and Mass Transfer PowerPoint Presentation, free Heat Transfer Q Units Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The heat flux can be ˙q determined by. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. Apply an equation to quantify the heat transfer that. Heat Transfer Q Units.
From www.alibaba.com
wholesale NFL Heat Transfer Designs Printable Plastisol DTF Vinyl Film Heat Transfer Q Units Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan,. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Sometimes it is important. Heat Transfer Q Units.
From heattransferkarikuse.blogspot.com
Heat Transfer Heat Transfer Q Equation Heat Transfer Q Units The quantitative relationship between heat transfer and temperature change contains all three factors: Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Use the equation for heat. Heat Transfer Q Units.
From pametno21.blogspot.com
Q=Mcat Formula Chemistry pametno Heat Transfer Q Units The heat flux can be ˙q determined by. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial. Heat Transfer Q Units.
From www.alibaba.com
High Quality custom size softball sport team Dtf Transfers Ready To Heat Transfer Q Units Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The quantitative relationship between heat transfer and temperature change contains all three factors: Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The primary objective of this.. Heat Transfer Q Units.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube Heat Transfer Q Units Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan,. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to. Heat Transfer Q Units.
From www.screenprinting.com
Quickfire Q&A Session Deep Dive With Colin by Heat Transfer Q Units Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The quantitative relationship between heat transfer and temperature change contains all three factors: The heat flux can be ˙q determined by. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the.. Heat Transfer Q Units.
From www.slideserve.com
PPT Conduction & Convection PowerPoint Presentation, free download Heat Transfer Q Units Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. The heat flux can be ˙q determined by. Sometimes it is important to determine the heat transfer. Heat Transfer Q Units.
From www.thermal-engineering.org
O que é condutividade térmica do vidro Definição Heat Transfer Q Units The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. The heat flux can. Heat Transfer Q Units.
From mungfali.com
Convective Heat Transfer Coefficient Units Heat Transfer Q Units Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The heat flux. Heat Transfer Q Units.
From www.slideshare.net
Thermodynamics Chapter 3 Heat Transfer Heat Transfer Q Units Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Q = mcδt, where q is the symbol for heat transfer, m is the. Heat Transfer Q Units.
From www.alibaba.com
Factory Custom Clothes Accessories Wholesale Heat Transfer Logo High Heat Transfer Q Units Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The primary objective of this. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. Heat transfer is a process in which the thermal energy of molecules is. Heat Transfer Q Units.
From www.alibaba.com
Personalised Polyester custom bright color sublimation heat transfer Heat Transfer Q Units Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. The quantitative relationship. Heat Transfer Q Units.
From www.alibaba.com
Custom Iron On DTF Transfer printing DIY company Logo Letter Text Heat Transfer Q Units The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The. Heat Transfer Q Units.
From www.slideserve.com
PPT Overall Heat Transfer Coefficient PowerPoint Presentation, free Heat Transfer Q Units The primary objective of this. The quantitative relationship between heat transfer and temperature change contains all three factors: Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature. Heat Transfer Q Units.
From www.chegg.com
The LMTD of a counter flow heat exchanger is 20°C. Heat Transfer Q Units The quantitative relationship between heat transfer and temperature change contains all three factors: Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan,. Sometimes it is important to determine the heat transfer. Heat Transfer Q Units.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi Heat Transfer Q Units Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. Experiments show that the heat transferred to or from a substance depends on three factors—the change in. Heat Transfer Q Units.
From www.chegg.com
Solved How to study for EXAM1 and Review INFORevised2.pdf Heat Transfer Q Units Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The primary objective of this. Apply an equation. Heat Transfer Q Units.
From www.tessshebaylo.com
Hvac Formulas & Equations Tessshebaylo Heat Transfer Q Units Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan,. Experiments show that the. Heat Transfer Q Units.
From www.numerade.com
SOLVED P5.16 Convection heat transfer data are often reported as a Heat Transfer Q Units The primary objective of this. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The quantitative relationship between heat transfer and temperature change contains all three factors: The heat flux. Heat Transfer Q Units.
From www.slideserve.com
PPT Heat Transfer Physical Origins and Rate Equations PowerPoint Heat Transfer Q Units Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The quantitative relationship between heat transfer and temperature change contains all three factors: The primary objective of this. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Q = mcδt,. Heat Transfer Q Units.
From www.youtube.com
Calculating Rate of Heat Transfer Through Composite Walls YouTube Heat Transfer Q Units Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The primary objective of this. The heat flux can be ˙q determined by. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Sometimes it is important to determine the heat. Heat Transfer Q Units.
From www.madebyteachers.com
Heat Transfer Calorimetry q = mCT First Law of Thermodynamics Worksheet Heat Transfer Q Units The heat flux can be ˙q determined by. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Heat transfer is a process in which the thermal energy of molecules is moved from the. Heat Transfer Q Units.
From library.fiveable.me
Energy, Work, and Heat Honors Chemistry Class Notes Fiveable Heat Transfer Q Units Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The heat flux. Heat Transfer Q Units.
From www.youtube.com
HTPIB14B Specific Heat and Q = mcT YouTube Heat Transfer Q Units The heat flux can be ˙q determined by. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The primary objective of this. Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the. Heat Transfer Q Units.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Heat Transfer Q Units Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. The primary objective of this. Heat transfer is a process in which the thermal energy of molecules is. Heat Transfer Q Units.
From www.alibaba.com
Custom Iron On DTF Transfer printing DIY company Logo Letter Text Heat Transfer Q Units Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The quantitative relationship between heat transfer and temperature change contains all three factors: The primary objective of this. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower. Heat Transfer Q Units.
From www.youtube.com
Calculating Rate of Heat Transfer Between Two Working Fluids of a Heat Heat Transfer Q Units The heat flux can be ˙q determined by. Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial. Heat Transfer Q Units.
From library.fiveable.me
Applications of Thermodynamics Heat Engines, Heat Pumps, and Heat Transfer Q Units Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. The heat flux can be ˙q determined by. The primary objective of this. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The quantitative relationship between. Heat Transfer Q Units.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Heat Transfer Q Units Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the. Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan,. Apply. Heat Transfer Q Units.
From www.youtube.com
Specific Heat Capacity q = mcT Everything you need to know! Chemistry Heat Transfer Q Units Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the. Heat Transfer Q Units.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free Heat Transfer Q Units Apply an equation to quantify the heat transfer that is associated with changing the temperature of a substance. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature. Heat Transfer Q Units.
From bazajustingraham.blogspot.com
Heat Transfer Coefficient Units Justin Graham Heat Transfer Q Units Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. The primary objective of this. Experiments show that the heat transferred to or from. Heat Transfer Q Units.