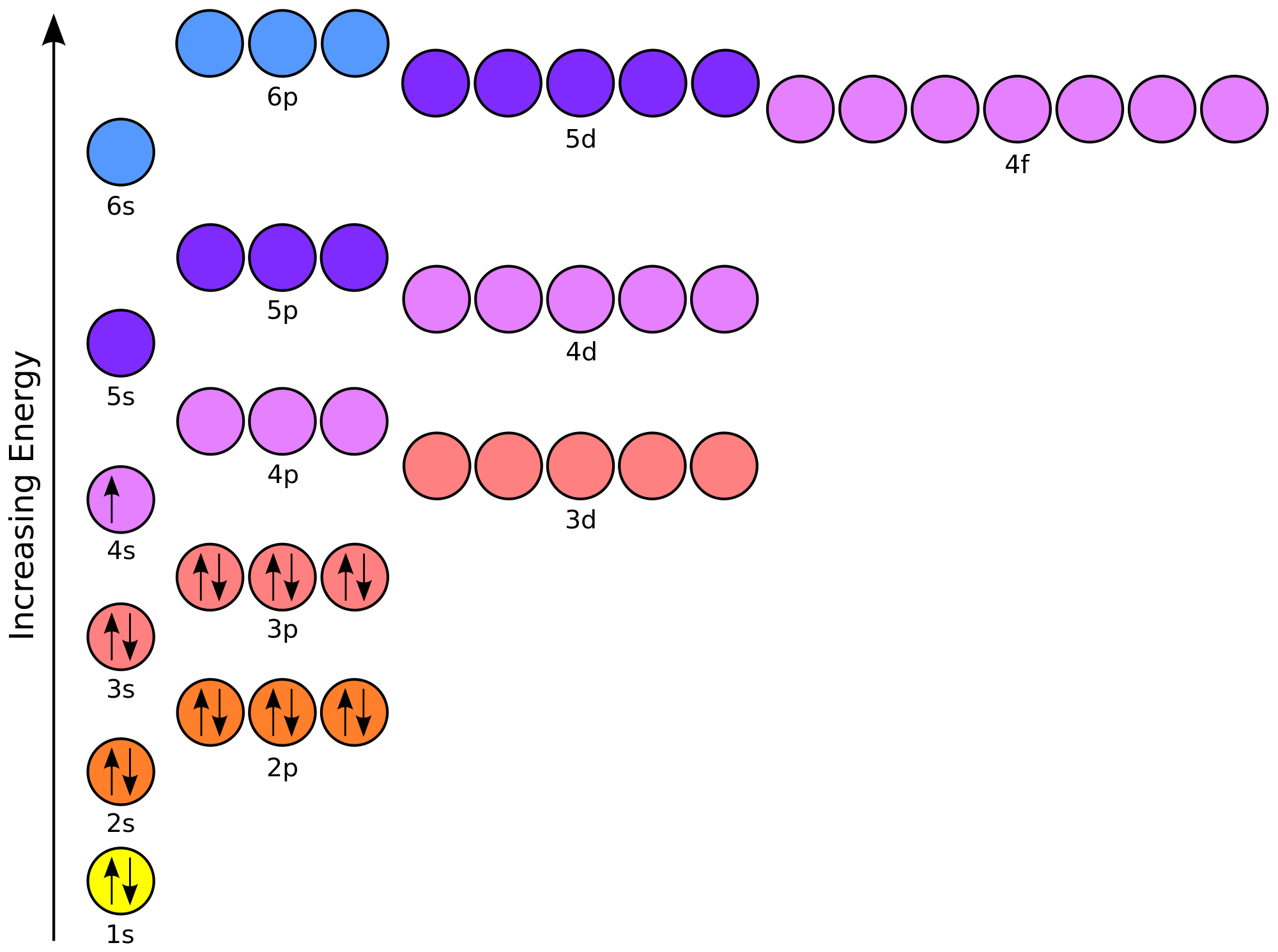
How Many Energy Levels Are Occupied In An Atom Of Bromine . This allows us to determine which orbitals are occupied by electrons in each atom. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. • the atoms in the second period. Electrons are tiny, negatively charged particles in. As a result, an electron in the 4p y orbital jumps to. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. When the bromine atom is excited, then the bromine atom absorbs energy.
from guidemanualeruptivity.z14.web.core.windows.net
Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. When the bromine atom is excited, then the bromine atom absorbs energy. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. As a result, an electron in the 4p y orbital jumps to. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Electrons are tiny, negatively charged particles in. • the atoms in the second period.
Rb Orbital Diagram
How Many Energy Levels Are Occupied In An Atom Of Bromine Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. When the bromine atom is excited, then the bromine atom absorbs energy. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electrons are tiny, negatively charged particles in. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. This allows us to determine which orbitals are occupied by electrons in each atom. It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. As a result, an electron in the 4p y orbital jumps to. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. • the atoms in the second period.
From proper-cooking.info
Bromine Atomic Structure How Many Energy Levels Are Occupied In An Atom Of Bromine Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. Electrons are tiny, negatively charged particles in. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.drawittoknowit.com
Biochemistry Glossary Orbitals 1. Occupancy & Energy Draw It to How Many Energy Levels Are Occupied In An Atom Of Bromine When the bromine atom is excited, then the bromine atom absorbs energy. This allows us to determine which orbitals are occupied by electrons in each atom. • the atoms in the second period. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. As a result, an electron in the 4p y orbital jumps. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From ar.inspiredpencil.com
Atom Energy Level Diagram How Many Energy Levels Are Occupied In An Atom Of Bromine The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. • the atoms in the second period. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.slideserve.com
PPT Properties of Atoms and the Periodic Table PowerPoint How Many Energy Levels Are Occupied In An Atom Of Bromine Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. As a result, an electron in the 4p y orbital jumps to. When the bromine atom is excited, then the. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From irpsiea4schematic.z21.web.core.windows.net
Schematic Energy Level Diagram How Many Energy Levels Are Occupied In An Atom Of Bromine As a result, an electron in the 4p y orbital jumps to. When the bromine atom is excited, then the bromine atom absorbs energy. Electrons are tiny, negatively charged particles in. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. Energy levels (also called electron shells) are fixed distances from the nucleus of. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.istockphoto.com
Atomic Structure Of Bromine With Atomic Number Atomic Mass And Energy How Many Energy Levels Are Occupied In An Atom Of Bromine Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. As a result, an electron in the 4p y orbital jumps to. • the atoms in the second period. Electrons are tiny, negatively charged particles in. Wavefunctions for each atom have some properties that are exact, for example each wavefunction. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.slideserve.com
PPT The Periodic Table & Its Properties Chapters 5 & 6 PowerPoint How Many Energy Levels Are Occupied In An Atom Of Bromine It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. This allows us to determine which orbitals are occupied by electrons in each atom. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic How Many Energy Levels Are Occupied In An Atom Of Bromine Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. This allows us to determine which orbitals are occupied by electrons in each atom. As a. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of How Many Energy Levels Are Occupied In An Atom Of Bromine As a result, an electron in the 4p y orbital jumps to. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. This allows us to determine which orbitals are occupied by electrons in each atom. Electron configuration notation provides us with information about the basic. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.slideserve.com
PPT Atomic Structure Atom Structure PowerPoint Presentation, free How Many Energy Levels Are Occupied In An Atom Of Bromine This allows us to determine which orbitals are occupied by electrons in each atom. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. When the bromine atom is excited, then the bromine atom absorbs energy. The specific arrangement of electrons in orbitals of an atom determines many of the. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.numerade.com
SOLVED What is the hybridization at bromine in [BrF4]+? Select one sp How Many Energy Levels Are Occupied In An Atom Of Bromine Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. When the bromine atom is excited, then the bromine atom absorbs energy. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. It is the pauli exclusion principle that requires. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps How Many Energy Levels Are Occupied In An Atom Of Bromine Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. As a result, an electron in the 4p y orbital jumps to. Energy levels (also called electron. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.quia.com
Quia Chap 6 The Periodic Table How Many Energy Levels Are Occupied In An Atom Of Bromine The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. This allows us to determine which orbitals are occupied by electrons in each atom. It is the pauli exclusion principle that. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From brainly.com
The highest energy occupied molecular orbital in the b−b bond of the b2 How Many Energy Levels Are Occupied In An Atom Of Bromine As a result, an electron in the 4p y orbital jumps to. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. When the bromine atom is excited, then the bromine atom absorbs energy. • the atoms in the second period. Number of energy levels in. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From giowenxdt.blob.core.windows.net
Ionization Energy Bromine Atom at Rueben Kraemer blog How Many Energy Levels Are Occupied In An Atom Of Bromine Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. The specific arrangement of. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.schoolmykids.com
Vanadium (V) Element Information, Facts, Properties, Uses Periodic How Many Energy Levels Are Occupied In An Atom Of Bromine When the bromine atom is excited, then the bromine atom absorbs energy. It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. The. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.vrogue.co
What Types Of Orbitals Are Found In The Principal Ene vrogue.co How Many Energy Levels Are Occupied In An Atom Of Bromine This allows us to determine which orbitals are occupied by electrons in each atom. It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. Electrons are tiny, negatively charged particles in. • the atoms in the second period. Energy levels (also called electron. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram How Many Energy Levels Are Occupied In An Atom Of Bromine As a result, an electron in the 4p y orbital jumps to. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. The specific arrangement of electrons in orbitals of. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.dreamstime.com
Magnesium Atom, with Mass and Energy Levels. Stock Vector How Many Energy Levels Are Occupied In An Atom Of Bromine It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. • the atoms in the second period. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. When the bromine atom is excited, then the bromine atom. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.dreamstime.com
Vanadium Atom, with Mass and Energy Levels. Stock Vector Illustration How Many Energy Levels Are Occupied In An Atom Of Bromine • the atoms in the second period. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. As a result, an electron in the 4p y orbital jumps to. This allows. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From mavink.com
Periodic Table With Energy Levels How Many Energy Levels Are Occupied In An Atom Of Bromine As a result, an electron in the 4p y orbital jumps to. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. This allows us to determine which orbitals are. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.youtube.com
1. Freely moving electrons in Solids (HSC Physics) YouTube How Many Energy Levels Are Occupied In An Atom Of Bromine Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. When the bromine atom is excited, then the bromine atom absorbs energy. As a result, an electron in the 4p. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From printablelibbroch.z21.web.core.windows.net
How To Determine Atomic Orbitals How Many Energy Levels Are Occupied In An Atom Of Bromine This allows us to determine which orbitals are occupied by electrons in each atom. Electrons are tiny, negatively charged particles in. As a result, an electron in the 4p y orbital jumps to. • the atoms in the second period. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Number of energy levels in. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From valenceelectrons.com
How many valence electrons does oxygen(O) have? How Many Energy Levels Are Occupied In An Atom Of Bromine • the atoms in the second period. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. When the bromine atom is excited,. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps How Many Energy Levels Are Occupied In An Atom Of Bromine This allows us to determine which orbitals are occupied by electrons in each atom. It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. When the bromine atom. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.museoinclusivo.com
Exploring How Many Energy Levels Does Aluminum Have Aluminum Profile Blog How Many Energy Levels Are Occupied In An Atom Of Bromine When the bromine atom is excited, then the bromine atom absorbs energy. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Electrons are tiny, negatively charged particles in. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. • the atoms in the second period. As a result,. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration How Many Energy Levels Are Occupied In An Atom Of Bromine It is the pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Electrons are tiny, negatively charged particles in. When the bromine atom is excited, then the bromine atom absorbs. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From guidemanualeruptivity.z14.web.core.windows.net
Rb Orbital Diagram How Many Energy Levels Are Occupied In An Atom Of Bromine As a result, an electron in the 4p y orbital jumps to. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. • the atoms in the second period. This allows us to determine which orbitals are occupied by electrons in each atom. Wavefunctions for each atom have some properties that are exact, for example. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From exoqqckld.blob.core.windows.net
How Many Energy Levels Are In Bromine at Frank Clemons blog How Many Energy Levels Are Occupied In An Atom Of Bromine Electrons are tiny, negatively charged particles in. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. Number of energy levels in each period • the atoms in the first period have electrons in 1 energy level. As a result, an electron in the 4p y. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of How Many Energy Levels Are Occupied In An Atom Of Bromine Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. As a result, an electron in the 4p y orbital jumps to. The specific arrangement of electrons in orbitals of an atom determines many of the chemical. It is the pauli exclusion principle that requires the. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.vrogue.co
Electron Dot Diagram For Br2 vrogue.co How Many Energy Levels Are Occupied In An Atom Of Bromine Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. This allows us to determine which orbitals are occupied by electrons in each atom. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. • the. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.sliderbase.com
Energy Levels, Sublevels, Electrons How Many Energy Levels Are Occupied In An Atom Of Bromine Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron in quantum state with a specific energy. Electrons are tiny, negatively charged particles in. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. As a result, an electron in the 4p y. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From www.gauthmath.com
Why is the atomic radius of iodine larger than the atomic radius of How Many Energy Levels Are Occupied In An Atom Of Bromine This allows us to determine which orbitals are occupied by electrons in each atom. Electrons are tiny, negatively charged particles in. When the bromine atom is excited, then the bromine atom absorbs energy. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Number of energy levels in each period. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br How Many Energy Levels Are Occupied In An Atom Of Bromine • the atoms in the second period. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons. Wavefunctions for each atom have some properties that are exact, for example each wavefunction describes an electron. How Many Energy Levels Are Occupied In An Atom Of Bromine.
From hubpages.com
Atoms and Atomic Structure HubPages How Many Energy Levels Are Occupied In An Atom Of Bromine The specific arrangement of electrons in orbitals of an atom determines many of the chemical. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. • the atoms in the second period. Number of energy levels in each period • the atoms in the first period have electrons in 1. How Many Energy Levels Are Occupied In An Atom Of Bromine.