Titration Indicator Color Change . You obviously need to choose. Instead, it actually undergoes a ph titration just. It is important to be aware that an indicator does not change color abruptly at a particular ph value; Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Indicators are substances whose solutions change color due to changes in ph. Here is a chart of common ph indicators, their ph range, their solutions,. They are usually weak acids or bases, but their conjugate base or acid. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances that change colour when they are added to acidic or alkaline solutions;
from slidetodoc.com
They are usually weak acids or bases, but their conjugate base or acid. Indicators are substances whose solutions change color due to changes in ph. You obviously need to choose. Here is a chart of common ph indicators, their ph range, their solutions,. When choosing the appropriate indicator, the ph of the equivalence point is very. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Indicators are substances that change colour when they are added to acidic or alkaline solutions; It is important to be aware that an indicator does not change color abruptly at a particular ph value; When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Instead, it actually undergoes a ph titration just.
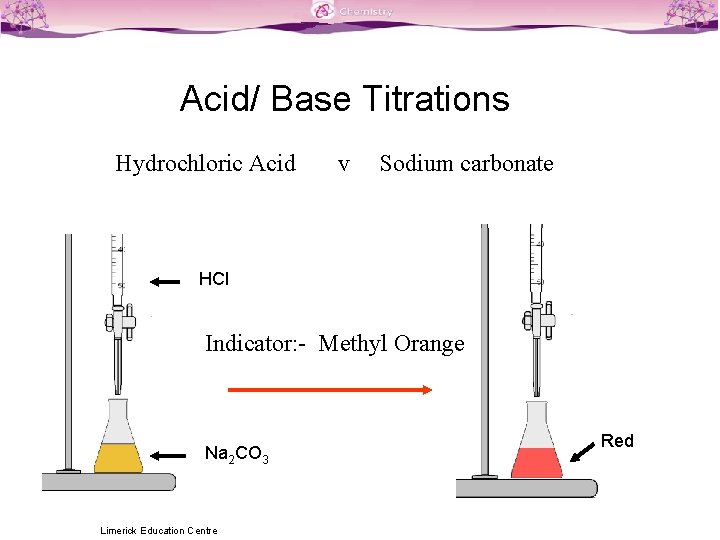
Titration Colour Changes SLSS Science Limerick Education Centre
Titration Indicator Color Change You obviously need to choose. They are usually weak acids or bases, but their conjugate base or acid. When choosing the appropriate indicator, the ph of the equivalence point is very. Here is a chart of common ph indicators, their ph range, their solutions,. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Instead, it actually undergoes a ph titration just. You obviously need to choose. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Indicators are substances that change colour when they are added to acidic or alkaline solutions; Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. It is important to be aware that an indicator does not change color abruptly at a particular ph value; Indicators are substances whose solutions change color due to changes in ph.
From fphoto.photoshelter.com
science chemistry acid base indicator methyl red Fundamental Titration Indicator Color Change Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. You obviously need to choose. Indicators are substances that change colour when they are added to acidic or alkaline solutions; Instead, it actually undergoes a ph titration just. It is important to be aware that an indicator does not change. Titration Indicator Color Change.
From www.slideshare.net
Acid base titration Titration Indicator Color Change Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. It is important to be aware that an indicator does not change color abruptly at a particular ph value; When choosing the. Titration Indicator Color Change.
From jackwestin.com
Indicators Titration MCAT Content Titration Indicator Color Change Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. You obviously need to choose. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Here is a chart of common ph indicators, their ph range, their solutions,. It is important to. Titration Indicator Color Change.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Titration Indicator Color Change Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. When choosing the appropriate indicator, the ph of the equivalence point is very. It is important to be aware that an indicator does not change color abruptly at a particular ph value; Indicators are substances that change colour when they. Titration Indicator Color Change.
From www.chegg.com
Solved Titration Indicators EXPERIMENT 1 GETTING AQUAINTED Titration Indicator Color Change It is important to be aware that an indicator does not change color abruptly at a particular ph value; When choosing the appropriate indicator, the ph of the equivalence point is very. You obviously need to choose. They are usually weak acids or bases, but their conjugate base or acid. Remember that the equivalence point of a titration is where. Titration Indicator Color Change.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Titration Indicator Color Change They are usually weak acids or bases, but their conjugate base or acid. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. It is important to be aware that an indicator does not change color abruptly at a particular ph value; Here is a chart of common ph indicators,. Titration Indicator Color Change.
From www.nagwa.com
Question Video Determining the Color of the Indicator Phenolphthalein Titration Indicator Color Change It is important to be aware that an indicator does not change color abruptly at a particular ph value; Here is a chart of common ph indicators, their ph range, their solutions,. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. You obviously need to choose. Instead, it actually. Titration Indicator Color Change.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Titration Indicator Color Change Instead, it actually undergoes a ph titration just. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Here is a chart of common ph indicators, their ph range, their solutions,. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances whose solutions change. Titration Indicator Color Change.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Indicator Color Change Instead, it actually undergoes a ph titration just. Indicators are substances whose solutions change color due to changes in ph. You obviously need to choose. They are usually weak acids or bases, but their conjugate base or acid. Indicators are substances that change colour when they are added to acidic or alkaline solutions; Remember that the equivalence point of a. Titration Indicator Color Change.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Titration Indicator Color Change Instead, it actually undergoes a ph titration just. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Indicators are substances that change colour when they are added to acidic or alkaline solutions; When titrating strong acids or bases, aim for a ph indicator that displays a color change near. Titration Indicator Color Change.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Indicator Color Change Indicators are substances that change colour when they are added to acidic or alkaline solutions; When choosing the appropriate indicator, the ph of the equivalence point is very. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Indicators are substances whose solutions change color due to changes in ph. It is. Titration Indicator Color Change.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Titration Indicator Color Change When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Indicators are substances that change colour when they are added to acidic or alkaline solutions; You obviously need to choose. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. They are. Titration Indicator Color Change.
From slideplayer.com
Titration Colour Changes ppt download Titration Indicator Color Change When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Indicators are substances whose solutions change color due to changes in ph. Here is a chart of common ph indicators, their ph range, their solutions,. Remember that the equivalence point of a titration is where you have mixed the two. Titration Indicator Color Change.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Indicator Color Change Indicators are substances that change colour when they are added to acidic or alkaline solutions; You obviously need to choose. Indicators are substances whose solutions change color due to changes in ph. Instead, it actually undergoes a ph titration just. Here is a chart of common ph indicators, their ph range, their solutions,. They are usually weak acids or bases,. Titration Indicator Color Change.
From ar.inspiredpencil.com
Titration Phenolphthalein Titration Indicator Color Change Instead, it actually undergoes a ph titration just. They are usually weak acids or bases, but their conjugate base or acid. Here is a chart of common ph indicators, their ph range, their solutions,. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. It is important to be aware that an. Titration Indicator Color Change.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Indicator Color Change Here is a chart of common ph indicators, their ph range, their solutions,. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. They are usually weak acids or bases, but their. Titration Indicator Color Change.
From giowagrjz.blob.core.windows.net
Indicator Titration Use at Virginia Mccracken blog Titration Indicator Color Change Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Here is a chart of common ph indicators, their ph range, their solutions,. You obviously need to choose. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. They are usually weak. Titration Indicator Color Change.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Titration Indicator Color Change When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Here is a chart. Titration Indicator Color Change.
From giowagrjz.blob.core.windows.net
Indicator Titration Use at Virginia Mccracken blog Titration Indicator Color Change You obviously need to choose. Indicators are substances that change colour when they are added to acidic or alkaline solutions; Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Here is a chart of common ph indicators, their ph range, their solutions,. It is important to be aware that. Titration Indicator Color Change.
From www.compoundchem.com
Compound Interest The Colours & Chemistry of pH Indicators Titration Indicator Color Change When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances that change colour when they are added to acidic or alkaline solutions; They are usually weak acids or bases, but their conjugate base or acid. You obviously need to choose. Instead, it actually undergoes a ph titration just. It is important to be aware. Titration Indicator Color Change.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Titration Indicator Color Change Indicators are substances whose solutions change color due to changes in ph. Instead, it actually undergoes a ph titration just. It is important to be aware that an indicator does not change color abruptly at a particular ph value; You obviously need to choose. They are usually weak acids or bases, but their conjugate base or acid. When titrating strong. Titration Indicator Color Change.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals Titration Indicator Color Change Indicators are substances that change colour when they are added to acidic or alkaline solutions; When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. They are usually weak acids. Titration Indicator Color Change.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 Titration Indicator Color Change Here is a chart of common ph indicators, their ph range, their solutions,. It is important to be aware that an indicator does not change color abruptly at a particular ph value; When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. They are usually weak acids or bases, but. Titration Indicator Color Change.
From saylordotorg.github.io
AcidBase Titrations Titration Indicator Color Change Here is a chart of common ph indicators, their ph range, their solutions,. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Indicators are substances whose solutions change color due to changes in ph. Remember that the equivalence point of a titration is where you have mixed the two substances in. Titration Indicator Color Change.
From gioxaqmhp.blob.core.windows.net
Indicators Titration at Lee Hughes blog Titration Indicator Color Change Instead, it actually undergoes a ph titration just. They are usually weak acids or bases, but their conjugate base or acid. Here is a chart of common ph indicators, their ph range, their solutions,. Indicators are substances whose solutions change color due to changes in ph. Remember that the equivalence point of a titration is where you have mixed the. Titration Indicator Color Change.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Titration Indicator Color Change They are usually weak acids or bases, but their conjugate base or acid. Here is a chart of common ph indicators, their ph range, their solutions,. You obviously need to choose. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Indicators are substances that change colour when they are added to. Titration Indicator Color Change.
From slideplayer.com
Titration Colour Changes ppt download Titration Indicator Color Change Indicators are substances whose solutions change color due to changes in ph. When choosing the appropriate indicator, the ph of the equivalence point is very. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Instead, it actually undergoes a ph titration just. Here is a chart of common ph. Titration Indicator Color Change.
From www.youtube.com
Titration end point using phenolphthalein indicator YouTube Titration Indicator Color Change It is important to be aware that an indicator does not change color abruptly at a particular ph value; Indicators are substances that change colour when they are added to acidic or alkaline solutions; Here is a chart of common ph indicators, their ph range, their solutions,. Remember that the equivalence point of a titration is where you have mixed. Titration Indicator Color Change.
From flatworldknowledge.lardbucket.org
Quantitative Analysis Using Titrations Titration Indicator Color Change Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. They are usually weak acids or bases, but their conjugate base or acid. You obviously need to choose. Instead, it actually undergoes a ph titration just. Remember that the equivalence point of a titration is where you have mixed the two substances. Titration Indicator Color Change.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Indicator Color Change When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. When choosing the appropriate indicator, the ph of the equivalence point is very. Here is a chart of common ph indicators, their ph range, their solutions,. Indicators are substances that change colour when they are added to acidic or alkaline. Titration Indicator Color Change.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations Titration Indicator Color Change Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid. Instead, it actually undergoes a ph titration just. You obviously need to choose. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. It is. Titration Indicator Color Change.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Titration Indicator Color Change Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid. Here is a chart of common ph indicators, their ph range, their solutions,. It is important to be aware that an indicator does not change color abruptly at a particular ph value; Indicators are substances. Titration Indicator Color Change.
From www.aiophotoz.com
Ph Indicator Chart Colors And Ranges Images and Photos finder Titration Indicator Color Change Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid. Instead, it actually undergoes a ph titration just. You obviously need to choose. Indicators are substances that change colour when they are added to acidic or alkaline solutions; Remember that the equivalence point of a. Titration Indicator Color Change.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Titration Indicator Color Change They are usually weak acids or bases, but their conjugate base or acid. Indicators are substances whose solutions change color due to changes in ph. You obviously need to choose. When choosing the appropriate indicator, the ph of the equivalence point is very. When titrating strong acids or bases, aim for a ph indicator that displays a color change near. Titration Indicator Color Change.
From mungfali.com
Acid Base Titration Indicator Titration Indicator Color Change Here is a chart of common ph indicators, their ph range, their solutions,. It is important to be aware that an indicator does not change color abruptly at a particular ph value; When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances that change colour when they are added to acidic or alkaline solutions;. Titration Indicator Color Change.