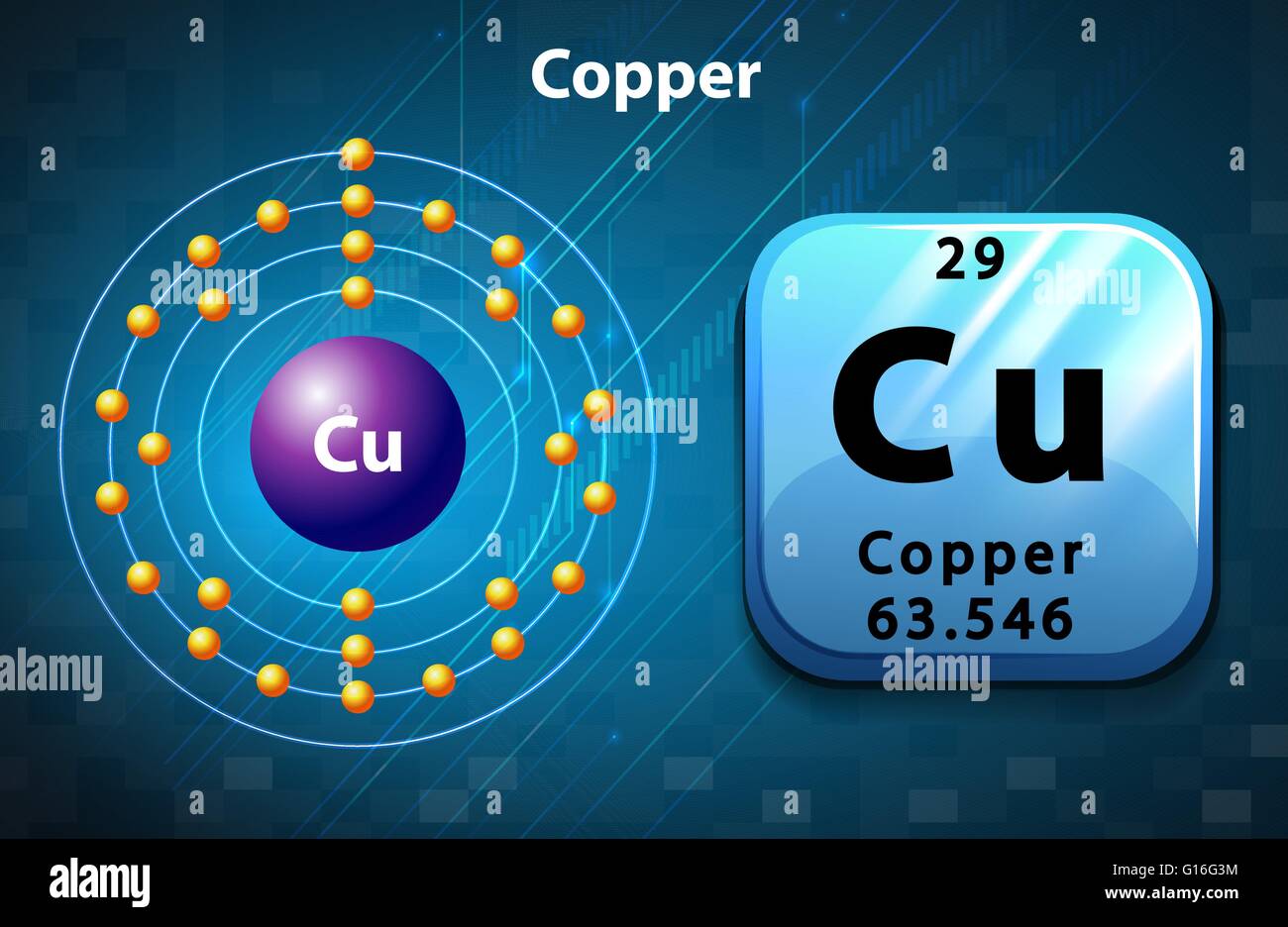
Copper # Of Electrons . The actual electron configuration of these. Possible oxidation states are +1,2. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. copper has the total of two valence electrons. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. electron configuration of copper is [ar] 3d10 4s1. Sources, facts, uses, scarcity (sri), podcasts,.
from www.alamy.com
Sources, facts, uses, scarcity (sri), podcasts,. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. electron configuration of copper is [ar] 3d10 4s1. Possible oxidation states are +1,2. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. The actual electron configuration of these. copper has the total of two valence electrons.
Copper atom hires stock photography and images Alamy
Copper # Of Electrons Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Possible oxidation states are +1,2. copper has the total of two valence electrons. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. Sources, facts, uses, scarcity (sri), podcasts,. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The actual electron configuration of these. electron configuration of copper is [ar] 3d10 4s1. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper # Of Electrons copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. electron configuration of copper is [ar] 3d10 4s1. copper has the total of two valence electrons. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The actual electron configuration. Copper # Of Electrons.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper # Of Electrons 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Possible oxidation states are +1,2. electron configuration of copper is [ar] 3d10 4s1. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and. Copper # Of Electrons.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper # Of Electrons 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. electron configuration of copper is [ar] 3d10 4s1. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to. Copper # Of Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Copper (Cu Copper # Of Electrons how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. copper is a. Copper # Of Electrons.
From www.dreamstime.com
Atom of Copper with Detailed Core and Its 29 Electrons on Black Stock Copper # Of Electrons how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The actual electron configuration of these. Sources, facts, uses, scarcity (sri), podcasts,. Possible oxidation states are +1,2. 1s 2 2s 2 2p 6 3 s 2. Copper # Of Electrons.
From www.alamy.com
Atom of Copper with Core and 29 Electrons with a black background Stock Copper # Of Electrons copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. copper has the total of two valence electrons. electron configuration of copper is [ar] 3d10 4s1. Possible oxidation states are. Copper # Of Electrons.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper # Of Electrons The actual electron configuration of these. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. copper has the total of two valence electrons. Possible oxidation states are +1,2. Copper forms a rich variety of. Copper # Of Electrons.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper # Of Electrons how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively.. Copper # Of Electrons.
From www.alamy.com
Copper atom hires stock photography and images Alamy Copper # Of Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Sources, facts, uses, scarcity (sri), podcasts,. copper has the total of. Copper # Of Electrons.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper # Of Electrons copper has the total of two valence electrons. Sources, facts, uses, scarcity (sri), podcasts,. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. electron configuration of copper is [ar] 3d10. Copper # Of Electrons.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper # Of Electrons Possible oxidation states are +1,2. The actual electron configuration of these. electron configuration of copper is [ar] 3d10 4s1. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. how to write the electron configuration. Copper # Of Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Copper # Of Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Sources, facts, uses, scarcity. Copper # Of Electrons.
From pnghut.com
Bohr Model Atom Electron Shell Copper Rutherford Elements Vector Copper # Of Electrons Possible oxidation states are +1,2. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Sources, facts, uses, scarcity (sri), podcasts,. The actual electron configuration. Copper # Of Electrons.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Copper # Of Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Sources, facts, uses, scarcity. Copper # Of Electrons.
From donghokiddy.com
Does Copper Really Have 3 Valence Electrons? Exploring Its Atomic Copper # Of Electrons Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. electron configuration of copper is [ar] 3d10 4s1. Possible oxidation states are +1,2. Sources, facts, uses, scarcity (sri), podcasts,. copper has the total of two valence electrons. The actual electron configuration of these. how to write the electron configuration for copper (cu, cu+, and cu2+) in. Copper # Of Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Copper # Of Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. The actual electron configuration of these. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. electron configuration of copper is [ar] 3d10 4s1. Sources, facts, uses, scarcity (sri), podcasts,. Copper’s electron. Copper # Of Electrons.
From childhealthpolicy.vumc.org
💐 Copper atom model. Copper Particles. 20221017 Copper # Of Electrons copper has the total of two valence electrons. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Possible oxidation states are +1,2. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of.. Copper # Of Electrons.
From www.earthdate.org
Copper’s Superpower EarthDate Copper # Of Electrons electron configuration of copper is [ar] 3d10 4s1. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Sources, facts, uses, scarcity (sri), podcasts,. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with. Copper # Of Electrons.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper # Of Electrons copper has the total of two valence electrons. electron configuration of copper is [ar] 3d10 4s1. Possible oxidation states are +1,2. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+,. Copper # Of Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Copper Have? Copper # Of Electrons The actual electron configuration of these. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Sources, facts, uses, scarcity (sri), podcasts,. copper has the total of two valence electrons. electron configuration of copper. Copper # Of Electrons.
From www.nuclear-power.com
Copper Electron Affinity Electronegativity Ionization Energy of Copper # Of Electrons electron configuration of copper is [ar] 3d10 4s1. The actual electron configuration of these. Sources, facts, uses, scarcity (sri), podcasts,. copper has the total of two valence electrons. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Possible oxidation states are +1,2. Copper’s electron configuration. Copper # Of Electrons.
From nice-electronic.blogspot.com
Copper 64 Protons Neutrons Electrons Copper # Of Electrons Sources, facts, uses, scarcity (sri), podcasts,. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. electron configuration of copper is [ar] 3d10 4s1. Possible oxidation states are +1,2. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron. Copper # Of Electrons.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper # Of Electrons copper has the total of two valence electrons. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The actual electron configuration of these. Possible oxidation states are +1,2. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. electron configuration of copper is [ar]. Copper # Of Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper # Of Electrons Possible oxidation states are +1,2. copper has the total of two valence electrons. electron configuration of copper is [ar] 3d10 4s1. Sources, facts, uses, scarcity (sri), podcasts,. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. The actual electron configuration of these. 1s 2 2s. Copper # Of Electrons.
From utedzz.blogspot.com
Periodic Table Copper Element Periodic Table Timeline Copper # Of Electrons Sources, facts, uses, scarcity (sri), podcasts,. The actual electron configuration of these. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. Possible oxidation states are +1,2. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. copper has the total of two valence electrons.. Copper # Of Electrons.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper # Of Electrons Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Possible oxidation states are +1,2. copper has the total of two valence electrons. Sources, facts, uses, scarcity (sri), podcasts,. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. electron configuration of copper is [ar] 3d10 4s1. how to write the. Copper # Of Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Copper # Of Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. electron configuration of copper is [ar] 3d10 4s1. 1s 2 2s 2 2p 6. Copper # Of Electrons.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper # Of Electrons The actual electron configuration of these. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Sources, facts, uses, scarcity (sri), podcasts,. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. how to write the electron configuration for copper (cu,. Copper # Of Electrons.
From stock.adobe.com
Cu Copper Element Information Facts, Properties, Trends, Uses and Copper # Of Electrons copper has the total of two valence electrons. Possible oxidation states are +1,2. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Sources, facts, uses, scarcity (sri), podcasts,. Copper forms a rich variety of. Copper # Of Electrons.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper # Of Electrons copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. copper has the total of two valence electrons. Sources, facts, uses, scarcity (sri), podcasts,. electron configuration of copper is [ar] 3d10 4s1. Possible oxidation states are +1,2. 1s 2 2s 2 2p 6 3 s. Copper # Of Electrons.
From www.alamy.com
Cu Copper Chemical Element Periodic Table. Single vector illustration Copper # Of Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Possible oxidation states are +1,2. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. copper has the total of two valence electrons. The. Copper # Of Electrons.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper # Of Electrons Possible oxidation states are +1,2. Sources, facts, uses, scarcity (sri), podcasts,. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. copper has the total of two valence electrons. electron configuration of copper is [ar] 3d10 4s1. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron. Copper # Of Electrons.
From www.dreamstime.com
Atom of Copper with Detailed Core and Its 29 Electrons with Atoms Stock Copper # Of Electrons Possible oxidation states are +1,2. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. The actual electron configuration of these. copper has the total of two valence electrons. electron configuration of copper is [ar]. Copper # Of Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper # Of Electrons Possible oxidation states are +1,2. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The actual electron configuration of these. copper is a. Copper # Of Electrons.
From sciencenotes.org
Copper Facts Cu or Atomic Number 29 Copper # Of Electrons Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Possible oxidation states are +1,2. The actual electron configuration of these. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which. Copper # Of Electrons.