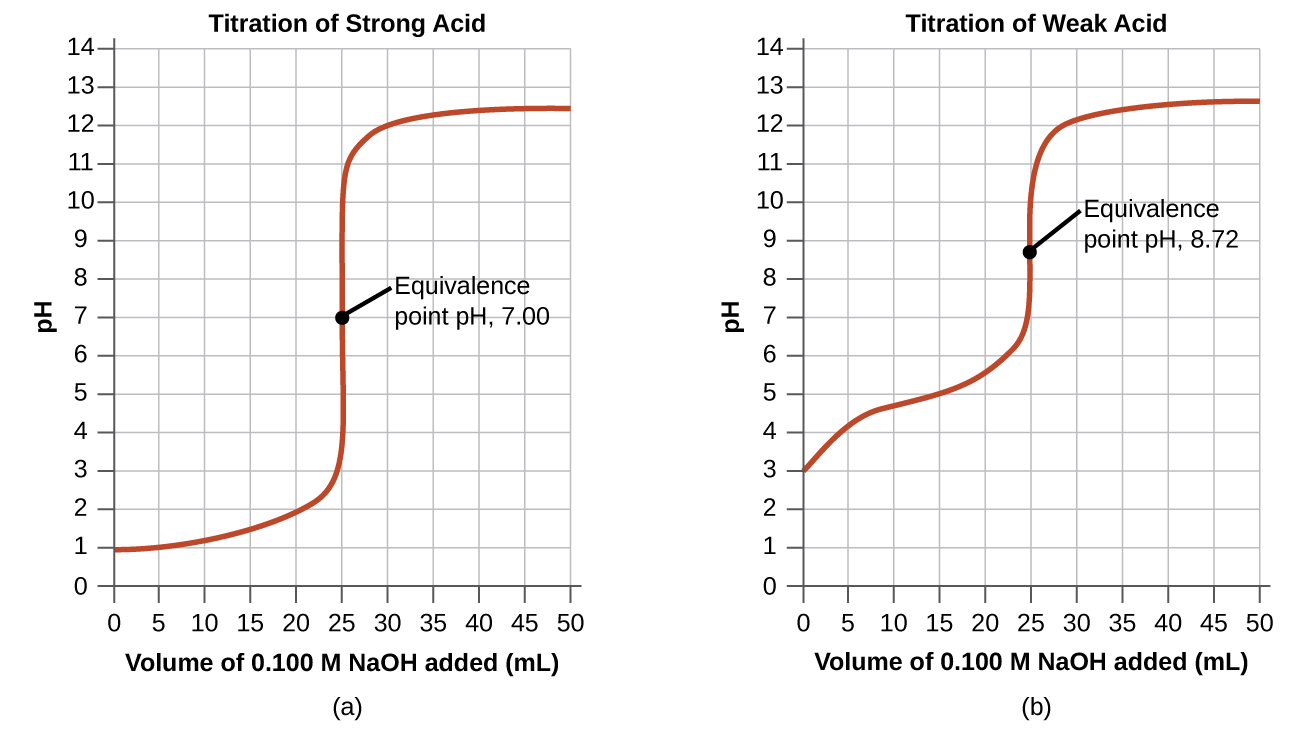
Acetic Acid Ph . Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Here's how you can do that. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Learn how to measure ph and what it means for acids and bases. For example, the ph of a 0.050 m acetic acid solution will be. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. The tool also explains the ph scale, the ph. Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration.
from
Here's how you can do that. Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Learn how to measure ph and what it means for acids and bases. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. For example, the ph of a 0.050 m acetic acid solution will be. The tool also explains the ph scale, the ph. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02.
Acetic Acid Ph The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Here's how you can do that. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. The tool also explains the ph scale, the ph. For example, the ph of a 0.050 m acetic acid solution will be. Learn how to measure ph and what it means for acids and bases.
From pubs.rsc.org
Control of pH by acetic acid and its effect on ethanol fermentation in Acetic Acid Ph Learn how to measure ph and what it means for acids and bases. Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. The tool also explains the ph scale, the ph. Calculated ph values of common acids and bases for 1, 10,. Acetic Acid Ph.
From
Acetic Acid Ph Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Here's how you can do that. Learn how to measure ph and what it means for acids and bases. For example, the ph of a 0.050 m acetic acid solution will be. Find out the ph values of. Acetic Acid Ph.
From
Acetic Acid Ph Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Ph =. Acetic Acid Ph.
From
Acetic Acid Ph For example, the ph of a 0.050 m acetic acid solution will be. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. Here's how you. Acetic Acid Ph.
From
Acetic Acid Ph Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. For. Acetic Acid Ph.
From
Acetic Acid Ph For example, the ph of a 0.050 m acetic acid solution will be. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Learn how to use this tool to find the ph of a solution from various. Acetic Acid Ph.
From
Acetic Acid Ph Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. For example, the ph of a 0.050 m acetic acid solution will be. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25,. Acetic Acid Ph.
From
Acetic Acid Ph Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. Learn how. Acetic Acid Ph.
From www.chegg.com
Solved Is the ionization of acetic acid a relatively Acetic Acid Ph The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard. Acetic Acid Ph.
From www.researchgate.net
Effect of different concentrations of acetic acid at different pH Acetic Acid Ph Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Learn how to use this tool to find the ph of. Acetic Acid Ph.
From sciencenotes.org
The pH Scale of Common Chemicals Acetic Acid Ph Here's how you can do that. For example, the ph of a 0.050 m acetic acid solution will be. The tool also explains the ph scale, the ph. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Learn how to use this tool to find the ph of a solution. Acetic Acid Ph.
From
Acetic Acid Ph Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. Learn how to measure ph and what it means for acids. Acetic Acid Ph.
From
Acetic Acid Ph Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Learn how to measure ph and what it means for acids and bases. The tool also explains the ph scale, the ph. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice,. Acetic Acid Ph.
From
Acetic Acid Ph The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. The tool also explains the ph scale, the ph. Learn how to measure ph. Acetic Acid Ph.
From
Acetic Acid Ph The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. For example, the ph of a 0.050 m acetic acid solution will be. Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant,. Acetic Acid Ph.
From
Acetic Acid Ph Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Find out the ph. Acetic Acid Ph.
From www.numerade.com
SOLVED (a) Calculate the pH of a 0.15M acetic acid solution. (b) You Acetic Acid Ph Here's how you can do that. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree. Acetic Acid Ph.
From
Acetic Acid Ph Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Learn how to measure ph and what it means for acids and bases. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. The tool also explains the ph. Acetic Acid Ph.
From
Acetic Acid Ph Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. Here's how you can do that. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; The tool also explains the. Acetic Acid Ph.
From www.researchgate.net
Production of lactic and acetic acids and pH values following 24 h Acetic Acid Ph Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Learn how to use this tool to find the ph of a solution from. Acetic Acid Ph.
From
Acetic Acid Ph Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. For example, the ph. Acetic Acid Ph.
From www.researchgate.net
pH at the MIC M s of acetic, butyric, citric, formic, lactic and Acetic Acid Ph Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. For example, the ph of a 0.050 m acetic acid solution will be. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon. Acetic Acid Ph.
From
Acetic Acid Ph Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. The tool also explains the ph scale, the ph. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its. Acetic Acid Ph.
From
Acetic Acid Ph Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. The tool also explains the ph scale, the ph. For example, the ph of a 0.050 m acetic acid solution will be. Learn how to calculate the ph of solutions of weak acids,. Acetic Acid Ph.
From
Acetic Acid Ph The tool also explains the ph scale, the ph. Learn how to measure ph and what it means for acids and bases. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. For example, the ph of a 0.050 m acetic acid solution will be. Find out the ph values of. Acetic Acid Ph.
From www.slideserve.com
PPT AcidBase Equilibria PowerPoint Presentation, free download ID Acetic Acid Ph Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Here's how you can do that. Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation,. Acetic Acid Ph.
From
Acetic Acid Ph The tool also explains the ph scale, the ph. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. Learn how to measure ph and what it means for acids and bases. For example, the ph of a 0.050 m acetic acid solution will be. Here's how you can do that. Calculated ph values of common acids and bases for. Acetic Acid Ph.
From www.chegg.com
Solved Determine the pH for the following solution of acetic Acetic Acid Ph Here's how you can do that. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. Calculated ph values of common acids and bases for 1, 10, and 100. Acetic Acid Ph.
From
Acetic Acid Ph Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Learn how to measure ph and what it means for acids and bases. Here's how you can do that. Find out the ph values of common chemicals, including vinegar (acetic acid),. Acetic Acid Ph.
From www.slideserve.com
PPT BCHS 3304 General Biochemistry I, Section 07553 Spring 2003 100 Acetic Acid Ph Learn how to measure ph and what it means for acids and bases. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. Calculated ph values of common acids. Acetic Acid Ph.
From
Acetic Acid Ph Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; The ph of acetic acid in water can range from approximately 2.4 to 4.3,. Acetic Acid Ph.
From
Acetic Acid Ph Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Learn how to calculate the ph of solutions of weak acids, bases, and salts using the equilibrium constant, the degree of dissociation, and the approximation of ka. Find out the ph values of common chemicals, including vinegar (acetic. Acetic Acid Ph.
From ar.inspiredpencil.com
Ph Scale Acids And Bases Acetic Acid Ph For example, the ph of a 0.050 m acetic acid solution will be. Ph = −log(0.050 ⋅ 1.8 ⋅ 10−5) ph = 3.02. The ph of acetic acid in water can range from approximately 2.4 to 4.3, depending on its molar concentration. Here's how you can do that. Learn how to use this tool to find the ph of a. Acetic Acid Ph.
From
Acetic Acid Ph For example, the ph of a 0.050 m acetic acid solution will be. Find out the ph values of common chemicals, including vinegar (acetic acid), lemon juice, and human blood. Learn how to use this tool to find the ph of a solution from various inputs, such as concentration, mass, volume, or ion concentration. Calculated ph values of common acids. Acetic Acid Ph.
From
Acetic Acid Ph Learn how to measure ph and what it means for acids and bases. The tool also explains the ph scale, the ph. Calculated ph values of common acids and bases for 1, 10, and 100 mmol/l (valid for standard conditions at 25, 1 atm; Learn how to calculate the ph of solutions of weak acids, bases, and salts using the. Acetic Acid Ph.