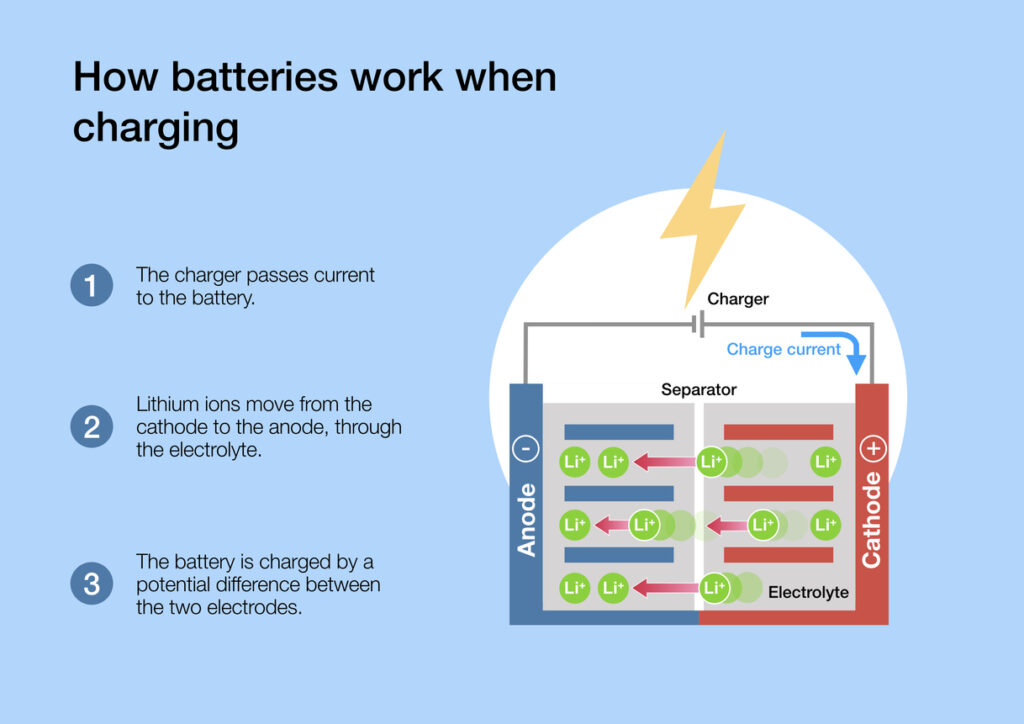
Electrodes Connected To Battery . There are three main components of a battery: In a battery cell we have two electrodes: Two terminals made of different chemicals (typically metals), the anode and the. There are two basic kinds of batteries: One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; This energy leaves the battery. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery.
from www.aquametals.com
When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. Two terminals made of different chemicals (typically metals), the anode and the. There are three main components of a battery: This energy leaves the battery. There are two basic kinds of batteries: In a battery cell we have two electrodes: When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts.
What Are Battery Anode and Cathode Materials? AquaMetals
Electrodes Connected To Battery At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. Two terminals made of different chemicals (typically metals), the anode and the. There are three main components of a battery: There are two basic kinds of batteries: One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. This energy leaves the battery. In a battery cell we have two electrodes: At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery.
From saylordotorg.github.io
Electrochemistry Electrodes Connected To Battery This energy leaves the battery. In a battery cell we have two electrodes: There are two basic kinds of batteries: When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. Disposable, or primary, batteries, in. Electrodes Connected To Battery.
From www.batterypowertips.com
What is a cathode? Battery Power Tips Electrodes Connected To Battery When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. In a battery cell we. Electrodes Connected To Battery.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrodes Connected To Battery At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. There are. Electrodes Connected To Battery.
From semcouniversity.com
How the three electrode system works Semco University Semco Electrodes Connected To Battery This energy leaves the battery. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. When you connect a battery's two electrodes. Electrodes Connected To Battery.
From pixels.com
Battery Connected To Two Electrodes Photograph by Dorling Kindersley Electrodes Connected To Battery There are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; This energy leaves the battery. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Two terminals made of different chemicals (typically metals), the. Electrodes Connected To Battery.
From www.mdpi.com
Batteries Free FullText Symmetric and Asymmetric Supercapacitors Electrodes Connected To Battery In a battery cell we have two electrodes: When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. There are three main components of a battery: Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; Two terminals made of different. Electrodes Connected To Battery.
From pineresearch.com
TwoElectrode Setups Pine Research Instrumentation Store Electrodes Connected To Battery There are three main components of a battery: This energy leaves the battery. In a battery cell we have two electrodes: When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. There are two basic kinds of batteries: Two terminals made of different chemicals (typically metals), the anode. Electrodes Connected To Battery.
From www.mdpi.com
Batteries Free FullText Critical Review of the Use of Reference Electrodes Connected To Battery One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. This energy leaves the battery. There are two basic. Electrodes Connected To Battery.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode Electrodes Connected To Battery Two terminals made of different chemicals (typically metals), the anode and the. There are two basic kinds of batteries: When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. There are three main components of. Electrodes Connected To Battery.
From www.chegg.com
Suppose a powerful battery is connected between a Electrodes Connected To Battery Two terminals made of different chemicals (typically metals), the anode and the. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. This energy leaves the battery. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. In a battery cell we. Electrodes Connected To Battery.
From diagramlibrarypern.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrodes Connected To Battery When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. There are three main components of a battery: In a battery cell we have two electrodes: Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; Two terminals made of different chemicals (typically metals), the anode and. Electrodes Connected To Battery.
From app.pandai.org
Electrolytic Cell Electrodes Connected To Battery Two terminals made of different chemicals (typically metals), the anode and the. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. In a battery cell we have two electrodes: There are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which. Electrodes Connected To Battery.
From byjus.com
In the circuit given as Fig., Boojho observed that copper is deposited Electrodes Connected To Battery There are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. When discharging a battery, the cathode is. Electrodes Connected To Battery.
From www.aquametals.com
What Are Battery Anode and Cathode Materials? AquaMetals Electrodes Connected To Battery Two terminals made of different chemicals (typically metals), the anode and the. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. In a battery cell we have two electrodes: This energy leaves the battery. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be. Electrodes Connected To Battery.
From www.shutterstock.com
850 Battery electrodes Stock Photos, Images & Photography Shutterstock Electrodes Connected To Battery At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. This energy leaves the battery. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; There are two basic kinds of batteries: When discharging a battery, the cathode is the positive electrode, at which. Electrodes Connected To Battery.
From wisc.pb.unizin.org
Day 41 Electrolysis; Commercial Batteries Chemistry 109, Fall 2020 Electrodes Connected To Battery When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. In a battery cell we have two electrodes: When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Two terminals made of different chemicals (typically metals), the anode and the. There are. Electrodes Connected To Battery.
From news.mit.edu
How electrodes charge and discharge MIT News Massachusetts Electrodes Connected To Battery When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Two terminals made of different chemicals (typically metals), the anode and the. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. When you connect a battery's two electrodes into a circuit (for example, when. Electrodes Connected To Battery.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Electrodes Connected To Battery When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. In a battery cell we have two electrodes: One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. There are two basic kinds of batteries:. Electrodes Connected To Battery.
From circuitdelgerf1.z21.web.core.windows.net
Cathode Charge In Electrolysis Electrodes Connected To Battery Two terminals made of different chemicals (typically metals), the anode and the. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; There are three main components of a battery: This energy leaves the battery. When you connect a battery's two electrodes into a circuit (for example, when you put one in a. Electrodes Connected To Battery.
From inside.lgensol.com
How to Make a Battery Step 1. Electrode Manufacturing Roll Pressing Electrodes Connected To Battery When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. There are two basic kinds of batteries: One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. Disposable, or primary, batteries, in which the electrode. Electrodes Connected To Battery.
From www.biolinscientific.com
Wettability of electrodes Electrode calendering in Liion batteries Electrodes Connected To Battery There are three main components of a battery: One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; When discharging a battery, the cathode. Electrodes Connected To Battery.
From www.britannica.com
Leadacid storage battery Britannica Electrodes Connected To Battery There are two basic kinds of batteries: One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. This energy leaves the battery.. Electrodes Connected To Battery.
From www.chegg.com
Solved Suppose a powerful battery is connected between a Electrodes Connected To Battery This energy leaves the battery. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Two terminals made of different chemicals (typically metals), the anode and the. There are. Electrodes Connected To Battery.
From www.autocar.co.uk
Carbon electrodes promise EV battery performance breakthrough Autocar Electrodes Connected To Battery This energy leaves the battery. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. Two terminals made of different chemicals (typically metals), the anode and the. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. In a battery cell we. Electrodes Connected To Battery.
From www.alamy.com
Liion battery diagram. Vector illustration. Rechargeable battery in Electrodes Connected To Battery This energy leaves the battery. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. There are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; At this point, there will be a fixed electric. Electrodes Connected To Battery.
From www.researchgate.net
Schematic Diagram of 3D Battery Electrodes (AD) Interdigitated (A Electrodes Connected To Battery One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. There are two basic kinds of batteries: At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. Two terminals made of different. Electrodes Connected To Battery.
From www.pinterest.ca
How to Turn a Potato Into a Battery Science Project Potato battery Electrodes Connected To Battery This energy leaves the battery. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; When you connect. Electrodes Connected To Battery.
From www.researchgate.net
Schematic illustration of a composite electrode in lithiumion Electrodes Connected To Battery At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. There are two basic kinds of batteries: This energy leaves the battery. When discharging a battery, the cathode is. Electrodes Connected To Battery.
From www.chegg.com
Solved Suppose a powerful battery is connected between a Electrodes Connected To Battery There are three main components of a battery: When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. One electrode, known as the cathode, connects to the positive end of the battery and is where. Electrodes Connected To Battery.
From www.chegg.com
Solved Suppose a powerful battery is connected between a Electrodes Connected To Battery There are three main components of a battery: When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. There are two basic kinds of batteries: Two terminals made of different chemicals (typically. Electrodes Connected To Battery.
From www.chegg.com
Solved 10. Suppose a powerful battery is connected between a Electrodes Connected To Battery Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. This energy leaves the battery. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight),. Electrodes Connected To Battery.
From www.researchgate.net
Three electrodes coin cell assembly. This cell is made to validate the Electrodes Connected To Battery Disposable, or primary, batteries, in which the electrode reactions are effectively irreversible and which cannot be recharged; This energy leaves the battery. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. In a battery cell we have two electrodes: At. Electrodes Connected To Battery.
From batteryworld.varta-automotive.com
How does a car battery work and how is it constructed? Electrodes Connected To Battery One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. In a battery cell we have two electrodes: When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts. At. Electrodes Connected To Battery.
From e-batterystore.com
Polarization and electromotive force of leadacid battery electrodes SLAB Electrodes Connected To Battery There are two basic kinds of batteries: When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Two terminals made of different chemicals (typically metals), the anode and the. In a battery cell we have two electrodes: At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of. Electrodes Connected To Battery.
From www.hioki.com
What are the Electrode Sheets that Greatly Affect the Quality of Electrodes Connected To Battery Two terminals made of different chemicals (typically metals), the anode and the. In a battery cell we have two electrodes: There are three main components of a battery: At this point, there will be a fixed electric potential difference between the two electrodes (terminals) of the battery. This energy leaves the battery. Disposable, or primary, batteries, in which the electrode. Electrodes Connected To Battery.