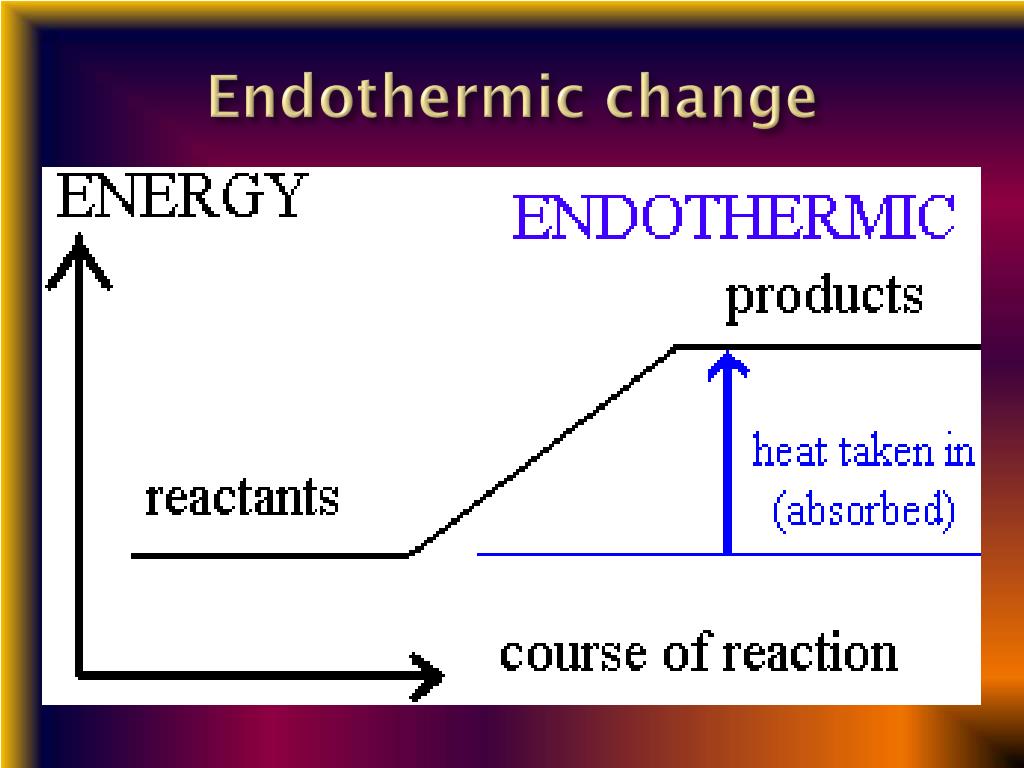
Phase Transitions Endothermic Or Exothermic . define phase transitions and phase transition temperatures; define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. changes of state are examples of phase changes, or phase transitions. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. Explain the relation between phase transition temperatures and. Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and intermolecular attractive forces.
from www.slideserve.com
All phase changes are accompanied by changes in the energy of a system. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures. define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and.
PPT Endothermic vs. Exothermic Reactions PowerPoint Presentation
Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and. define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures; Explain the relation between phase transition temperatures and intermolecular attractive forces. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and intermolecular attractive forces.
From opentextbc.ca
Phase Changes Basic HVAC Phase Transitions Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. All phase changes are accompanied by changes in the energy of a system. Explain the relation between phase transition temperatures and. Explain the relation between phase transition temperatures and intermolecular attractive forces. changes of state are examples of phase changes, or phase transitions. at the. Phase Transitions Endothermic Or Exothermic.
From kunduz.com
[ANSWERED] For each phase transition is the process endothermic or Kunduz Phase Transitions Endothermic Or Exothermic at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. Explain the relation between phase transition temperatures and intermolecular attractive forces. changes of state are examples of phase changes, or phase transitions. changes of state are examples of phase changes, or. Phase Transitions Endothermic Or Exothermic.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Phase Transitions Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and intermolecular attractive forces. changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. Explain the relation between. Phase Transitions Endothermic Or Exothermic.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Phase Transitions Endothermic Or Exothermic Explain the relation between phase transition temperatures and intermolecular attractive forces. All phase changes are accompanied by changes in the energy of a system. All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and. changes of state are examples of. Phase Transitions Endothermic Or Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Phase Transitions Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures; define phase transitions and phase transition temperatures. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. All phase changes are accompanied. Phase Transitions Endothermic Or Exothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Phase Transitions Endothermic Or Exothermic Explain the relation between phase transition temperatures and. All phase changes are accompanied by changes in the energy of a system. Explain the relation between phase transition temperatures and intermolecular attractive forces. changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures. define phase transitions and phase transition temperatures;. Phase Transitions Endothermic Or Exothermic.
From www.youtube.com
Lecture 1.11 exothermic, endothermic, phase changes, YouTube Phase Transitions Endothermic Or Exothermic Explain the relation between phase transition temperatures and intermolecular attractive forces. define phase transitions and phase transition temperatures. changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures; All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures.. Phase Transitions Endothermic Or Exothermic.
From slideplayer.com
Energy Changes & Phase Changes ppt download Phase Transitions Endothermic Or Exothermic Explain the relation between phase transition temperatures and intermolecular attractive forces. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. . Phase Transitions Endothermic Or Exothermic.
From studylibraryimburse.z22.web.core.windows.net
Endothermic Vs Exothermic Examples Chemistry Phase Transitions Endothermic Or Exothermic Explain the relation between phase transition temperatures and intermolecular attractive forces. define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. All phase changes are accompanied by changes in the energy of a system. at the phase transition, such as the boiling point between liquid and gas phases,. Phase Transitions Endothermic Or Exothermic.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and intermolecular attractive forces. changes of state are examples of phase changes, or phase transitions. at the phase transition, such as the boiling point between liquid and gas phases, the two. Phase Transitions Endothermic Or Exothermic.
From slideplayer.com
Chemistry Notes Phases of Matter ppt download Phase Transitions Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures. define phase transitions and phase transition temperatures; Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and.. Phase Transitions Endothermic Or Exothermic.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Phase Transitions Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. Explain the relation between phase transition temperatures and intermolecular attractive forces. changes of state are examples of phase changes, or phase transitions. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies. Phase Transitions Endothermic Or Exothermic.
From studylibraryimburse.z22.web.core.windows.net
Energy Diagram Chemistry Explained Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures; Explain the relation between phase transition temperatures and intermolecular attractive forces. All phase changes are accompanied by changes in the energy of a. Phase Transitions Endothermic Or Exothermic.
From www.researchgate.net
Endothermic or exothermic reaction of the VO 2 phase caused by phase Phase Transitions Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. changes of state are examples of phase changes, or phase transitions. Explain. Phase Transitions Endothermic Or Exothermic.
From pamelakruwhiggins.blogspot.com
Which of the Following Phase Changes Is an Endothermic Change Phase Transitions Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. Explain the relation between phase transition temperatures and. Phase Transitions Endothermic Or Exothermic.
From printableabsolutefreakmn.z14.web.core.windows.net
Endothermic And Exothermic Explained Phase Transitions Endothermic Or Exothermic at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. All phase changes are accompanied by changes in the energy of a system. Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and. Explain. Phase Transitions Endothermic Or Exothermic.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Phase Transitions Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. Explain the relation between phase transition temperatures and intermolecular attractive forces. define phase transitions and phase transition temperatures. All phase. Phase Transitions Endothermic Or Exothermic.
From www.slideshare.net
Unit 1 Presentation Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and. define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and intermolecular attractive forces. define phase transitions and phase transition temperatures; at the phase transition, such. Phase Transitions Endothermic Or Exothermic.
From lessonfullexcerption.z19.web.core.windows.net
Endothermic And Exothermic Explained Phase Transitions Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures; All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and intermolecular attractive forces. define phase. Phase Transitions Endothermic Or Exothermic.
From userlistnegligible.z13.web.core.windows.net
Phase Diagrams Understanding The Basics Phase Transitions Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures; Explain the relation between phase transition temperatures and intermolecular attractive forces. define phase transitions and phase transition temperatures. define phase transitions and phase transition temperatures.. Phase Transitions Endothermic Or Exothermic.
From thechemistrynotes.com
Differences Between Exothermic and Endothermic Reactions Phase Transitions Endothermic Or Exothermic Explain the relation between phase transition temperatures and intermolecular attractive forces. All phase changes are accompanied by changes in the energy of a system. Explain the relation between phase transition temperatures and intermolecular attractive forces. All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions.. Phase Transitions Endothermic Or Exothermic.
From www.slideserve.com
PPT Thermochemical Equations PowerPoint Presentation, free download Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. changes of state are examples of phase changes, or phase transitions. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. define phase transitions and phase transition temperatures; define phase transitions and. Phase Transitions Endothermic Or Exothermic.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Phase Transitions Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and intermolecular attractive forces. define phase transitions and phase transition temperatures. define phase transitions and phase transition temperatures; All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase. Phase Transitions Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Phase Transitions Endothermic Or Exothermic Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and intermolecular attractive forces. changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and. define phase transitions and phase transition temperatures; at. Phase Transitions Endothermic Or Exothermic.
From www.youtube.com
How to Determine if Phase Change is Endothermic or Exothermic Examples Phase Transitions Endothermic Or Exothermic at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. define phase transitions and phase transition temperatures; changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures. All phase changes are accompanied. Phase Transitions Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic vs. Exothermic Reactions PowerPoint Presentation Phase Transitions Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. Explain the relation between phase transition temperatures and intermolecular attractive forces. . Phase Transitions Endothermic Or Exothermic.
From www.thoughtco.com
List of Phase Changes Between States of Matter Phase Transitions Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. define phase transitions and phase transition temperatures.. Phase Transitions Endothermic Or Exothermic.
From ar.inspiredpencil.com
Endothermic And Exothermic Phase Changes Phase Transitions Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and. Explain the relation between phase transition temperatures and intermolecular attractive forces. define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and intermolecular attractive forces. at the phase transition, such as the boiling point. Phase Transitions Endothermic Or Exothermic.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. define phase transitions and phase transition temperatures; define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and intermolecular attractive forces. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and. Phase Transitions Endothermic Or Exothermic.
From ar.inspiredpencil.com
Endothermic And Exothermic Phase Changes Phase Transitions Endothermic Or Exothermic at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system. Explain the relation between phase transition temperatures and. Phase Transitions Endothermic Or Exothermic.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Phase Transitions Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and. Explain the relation between phase transition temperatures and intermolecular attractive forces. at the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. define. Phase Transitions Endothermic Or Exothermic.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and. at the phase transition, such as the boiling point. Phase Transitions Endothermic Or Exothermic.
From whatsinsight.org
Endothermic vs. Exothermic with Examples What's Insight Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. Explain the relation between phase transition temperatures and. All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. define phase transitions and phase transition temperatures. changes of state are examples of phase changes, or phase. Phase Transitions Endothermic Or Exothermic.
From guidelibgatherings.z21.web.core.windows.net
Exothermic And Endothermic Energy Diagrams Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures; Explain the relation between phase transition temperatures and intermolecular attractive forces. Explain the relation between phase transition temperatures and. at the phase transition, such as the boiling point between liquid. Phase Transitions Endothermic Or Exothermic.
From fity.club
Comparing Endothermic And Exothermic Potential Energy Phase Transitions Endothermic Or Exothermic define phase transitions and phase transition temperatures. All phase changes are accompanied by changes in the energy of a system. define phase transitions and phase transition temperatures. define phase transitions and phase transition temperatures; changes of state are examples of phase changes, or phase transitions. Explain the relation between phase transition temperatures and. All phase changes. Phase Transitions Endothermic Or Exothermic.