Standard Heat Of Formation Cocl2 . The list is limited to 20 most. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the.
from www.chegg.com
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The list is limited to 20 most.
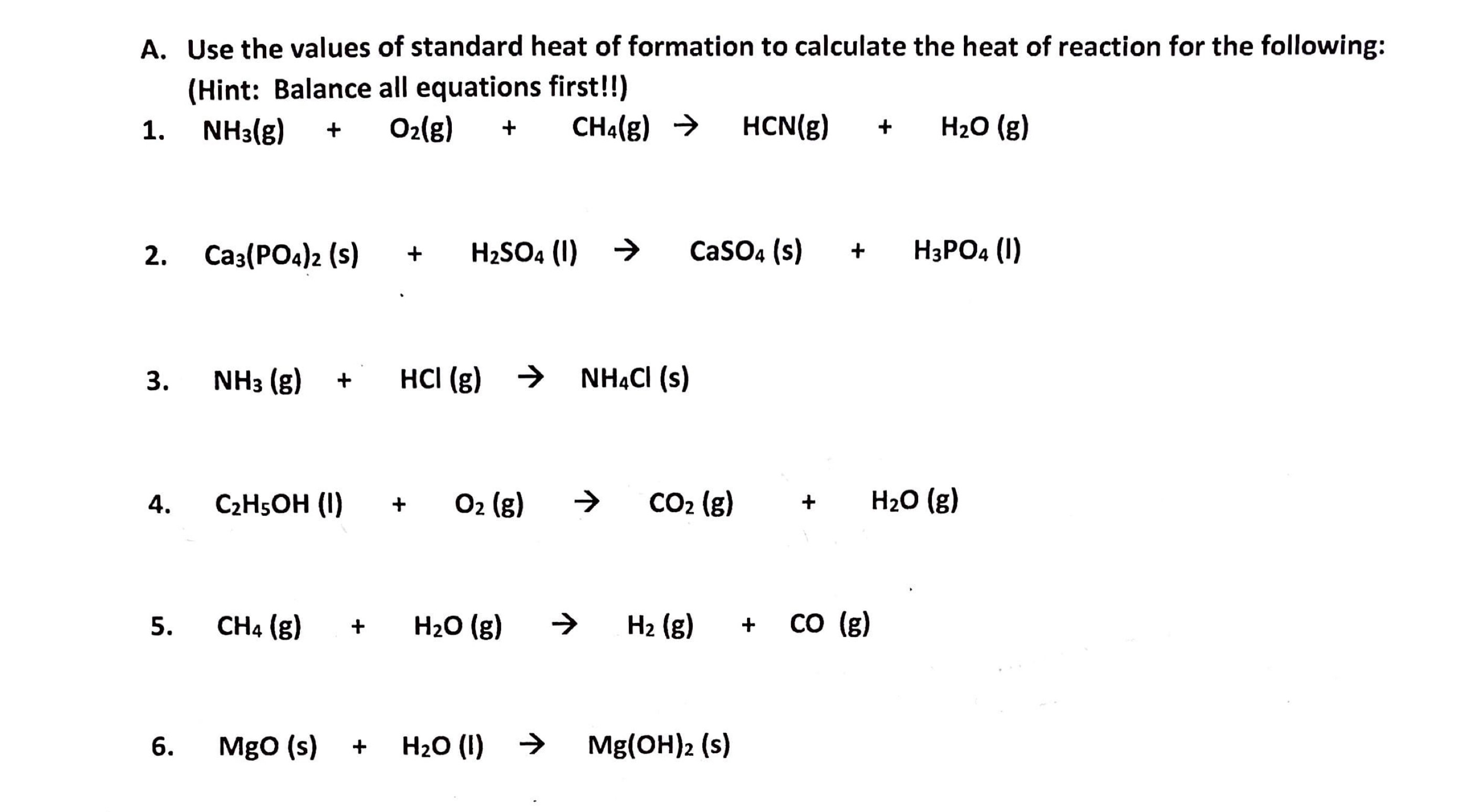
Solved A. Use the values of standard heat of formation to
Standard Heat Of Formation Cocl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The list is limited to 20 most.
From www.showme.com
11.4Calculating Heat ChangesStandard Heats of Formation (II) Science Standard Heat Of Formation Cocl2 The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Standard Heat Of Formation Cocl2.
From ar.inspiredpencil.com
Heat Of Formation Table Standard Heat Of Formation Cocl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The list is limited to 20 most. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation is a. Standard Heat Of Formation Cocl2.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Heat Of Formation Cocl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include. Standard Heat Of Formation Cocl2.
From printablevascelomgm.z13.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Cocl2 The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Heat Of Formation Cocl2.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Cocl2 The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 136 rows standard enthalpy change of formation (data table) these tables include. Standard Heat Of Formation Cocl2.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Cocl2 The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved Use and interpret standard heats of formation. (a) Standard Heat Of Formation Cocl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The list is limited to 20 most. 136 rows standard enthalpy change. Standard Heat Of Formation Cocl2.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Of Formation Cocl2 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The 3 contributors listed below account for 92.5% of the provenance of. Standard Heat Of Formation Cocl2.
From byjus.com
The standard molar heat for formation ofethane, carbondioxide and water Standard Heat Of Formation Cocl2 The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of. Standard Heat Of Formation Cocl2.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Cocl2 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Heat Of Formation Cocl2.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard Standard Heat Of Formation Cocl2 The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1. Standard Heat Of Formation Cocl2.
From learningschoolandy.z21.web.core.windows.net
Heat Of Formation List Standard Heat Of Formation Cocl2 The list is limited to 20 most. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation, δh ∘ f, is the enthalpy change. Standard Heat Of Formation Cocl2.
From learningfullproceed.z21.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Cocl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved The standard heat of formation for CaCl2 (s) is −796 Standard Heat Of Formation Cocl2 The list is limited to 20 most. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. 193 rows in chemistry and thermodynamics, the. Standard Heat Of Formation Cocl2.
From www.numerade.com
SOLVED Formation Reactions Review Constants Periodic Table The Standard Heat Of Formation Cocl2 The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The list is limited to 20 most. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 193 rows in chemistry and. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved The standard heat of formation, ΔH∘f, is defined as Standard Heat Of Formation Cocl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, δh ∘ f, is the enthalpy change. Standard Heat Of Formation Cocl2.
From studylib.net
heats of formation worksheet key Standard Heat Of Formation Cocl2 The list is limited to 20 most. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved 19. Use the given information to calculate the Standard Heat Of Formation Cocl2 The list is limited to 20 most. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The 3 contributors listed below. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved What Is Delta Hrxn Theta For The Following Chemica... Standard Heat Of Formation Cocl2 The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved A. Use the values of standard heat of formation to Standard Heat Of Formation Cocl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, δh ∘ f, is the enthalpy change. Standard Heat Of Formation Cocl2.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Standard Heat Of Formation Cocl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The list is limited to 20 most. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. 136 rows standard enthalpy change of formation. Standard Heat Of Formation Cocl2.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Cocl2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Heat Of Formation Cocl2.
From www.researchgate.net
Heat capacity of CoCl2‧6H2O in the vicinity of the ordering Standard Heat Of Formation Cocl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The list is limited to 20 most. 136 rows standard enthalpy change. Standard Heat Of Formation Cocl2.
From www.youtube.com
Standard heat of formation problem / Heat of formation formation Standard Heat Of Formation Cocl2 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Heat Of Formation Cocl2.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Cocl2 The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1. Standard Heat Of Formation Cocl2.
From www.numerade.com
SOLVED Text DIOPLOIDOUEHLSUPISUOLMDWOH What is the enthalpy change Standard Heat Of Formation Cocl2 The list is limited to 20 most. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Heat Of Formation Cocl2.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Heat Of Formation Cocl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Heat Of Formation Cocl2.
From lessonschoolwinchell.z21.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Cocl2 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Heat Of Formation Cocl2.
From www.numerade.com
SOLVED Using the provided table and the equation below, determine the Standard Heat Of Formation Cocl2 The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved The standard heat of formation for BaO(s) is −554 Standard Heat Of Formation Cocl2 The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved The standard heat of formation for CaCl2 (s) is −796 Standard Heat Of Formation Cocl2 The list is limited to 20 most. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The 3 contributors listed below. Standard Heat Of Formation Cocl2.
From www.chegg.com
Solved The standard heat of formation, ΔHf, is defined as Standard Heat Of Formation Cocl2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Heat Of Formation Cocl2.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Cocl2 The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The list is limited to 20 most. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The 3 contributors listed below. Standard Heat Of Formation Cocl2.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Standard Heat Of Formation Cocl2 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The list is limited to 20 most. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). The standard enthalpy of formation is a measure of the energy released. Standard Heat Of Formation Cocl2.
From www.studypool.com
SOLUTION Heat Of Formation Studypool Standard Heat Of Formation Cocl2 The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The 3 contributors listed below account for 92.5% of the provenance of δfh° of cocl2 (cr, l, ii+liquid). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Heat Of Formation Cocl2.