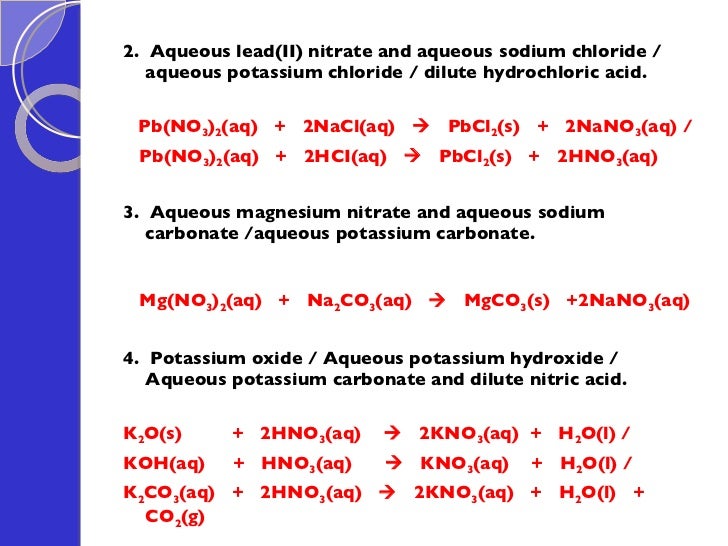
Lead Carbonate With Nitric Acid Reaction . experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. the reaction between copper(ii) carbonate and dilute sulfuric acid. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Here lead is oxidized to its +2. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. reactions of acids with carbonates. lead has no noticeable reaction with either of the dilute acids because lead (ii) chloride and lead (ii) sulfate are insoluble in water. lead and nitric acid reaction. , water and carbon dioxide are produced when acids react with carbonates. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. Copper(ii) carbonate is an insoluble green powder.
from www.slideshare.net
the reaction between copper(ii) carbonate and dilute sulfuric acid. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. Here lead is oxidized to its +2. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. lead has no noticeable reaction with either of the dilute acids because lead (ii) chloride and lead (ii) sulfate are insoluble in water. reactions of acids with carbonates. lead and nitric acid reaction. , water and carbon dioxide are produced when acids react with carbonates. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon.
Acids And Bases
Lead Carbonate With Nitric Acid Reaction lead and nitric acid reaction. , water and carbon dioxide are produced when acids react with carbonates. Here lead is oxidized to its +2. lead has no noticeable reaction with either of the dilute acids because lead (ii) chloride and lead (ii) sulfate are insoluble in water. reactions of acids with carbonates. the reaction between copper(ii) carbonate and dilute sulfuric acid. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. lead and nitric acid reaction. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Copper(ii) carbonate is an insoluble green powder.
From www.coursehero.com
[Solved] The correct net ionic equation for the reaction between nitric Lead Carbonate With Nitric Acid Reaction lead and nitric acid reaction. reactions of acids with carbonates. Here lead is oxidized to its +2. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Copper(ii) carbonate is an insoluble green powder. lead has no noticeable reaction with either of the dilute acids because lead (ii) chloride and lead (ii) sulfate. Lead Carbonate With Nitric Acid Reaction.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Lead Carbonate With Nitric Acid Reaction reactions of acids with carbonates. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. the reaction between copper(ii) carbonate and dilute sulfuric acid. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and. Lead Carbonate With Nitric Acid Reaction.
From www.numerade.com
SOLVED M02 Write the molecular; total ionic, and net ionic equation Lead Carbonate With Nitric Acid Reaction experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. the reaction between copper(ii) carbonate and dilute sulfuric acid. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. reactions of acids with carbonates. lead has no noticeable reaction with either of the dilute acids because lead (ii). Lead Carbonate With Nitric Acid Reaction.
From pubs.acs.org
Reactions of Metals in Nitric Acid Writing Equations and Calculating Lead Carbonate With Nitric Acid Reaction reactions of acids with carbonates. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. Copper(ii) carbonate is an insoluble green powder.. Lead Carbonate With Nitric Acid Reaction.
From exondqbhu.blob.core.windows.net
Lead Carbonate Atoms at Luke Crary blog Lead Carbonate With Nitric Acid Reaction (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. Here lead is oxidized to its +2. , water and carbon dioxide are produced when acids react with carbonates. experiments in this. Lead Carbonate With Nitric Acid Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Carbonate With Nitric Acid Reaction , water and carbon dioxide are produced when acids react with carbonates. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. reactions of acids with carbonates. Copper(ii) carbonate is an insoluble. Lead Carbonate With Nitric Acid Reaction.
From www.chemicals.co.uk
The Surprising Reaction Of Nitric Acid & Ammonia The Chemistry Blog Lead Carbonate With Nitric Acid Reaction Here lead is oxidized to its +2. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. , water and carbon dioxide are produced when acids react with carbonates. lead when reacted with. Lead Carbonate With Nitric Acid Reaction.
From www.slideshare.net
Presentation Nitric Acid Lead Carbonate With Nitric Acid Reaction lead and nitric acid reaction. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. reactions of acids with carbonates. . Lead Carbonate With Nitric Acid Reaction.
From www.masterorganicchemistry.com
Nitration and Sulfonation Reactions In Electrophilic Aromatic Substitution Lead Carbonate With Nitric Acid Reaction when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. lead and nitric acid reaction.. Lead Carbonate With Nitric Acid Reaction.
From www.numerade.com
SOLVED Am asking on how a solid sample of lead ii chloride can be Lead Carbonate With Nitric Acid Reaction (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. Here lead is oxidized to its +2. . Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
Nitric Acid What Happens When HNO3 acid is heated Lead Carbonate With Nitric Acid Reaction reactions of acids with carbonates. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. lead and nitric acid reaction. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide. Lead Carbonate With Nitric Acid Reaction.
From www.slideserve.com
PPT Types of chemical reactions STUDY GUIDE V2.0 PowerPoint Lead Carbonate With Nitric Acid Reaction Copper(ii) carbonate is an insoluble green powder. , water and carbon dioxide are produced when acids react with carbonates. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
Reaction of Nitric Acid with Carbonates and Bicarbonates, Chemistry Lead Carbonate With Nitric Acid Reaction experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. the reaction between copper(ii) carbonate and dilute sulfuric acid. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. Copper(ii) carbonate is an insoluble green powder. reactions of acids with carbonates. . Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Sodium Carbonate YouTube Lead Carbonate With Nitric Acid Reaction reactions of acids with carbonates. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. lead and nitric acid reaction. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water.. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
Reactions between Metal Carbonates and Acids YouTube Lead Carbonate With Nitric Acid Reaction Here lead is oxidized to its +2. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. the reaction between copper(ii) carbonate and dilute sulfuric acid. lead and nitric acid reaction. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Lead(pb) and dilute nitric acid react to. Lead Carbonate With Nitric Acid Reaction.
From www.numerade.com
SOLVED The reaction of nitric acid with calcium carbonate produces Lead Carbonate With Nitric Acid Reaction Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. Here lead is oxidized to its +2. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. lead has no noticeable reaction with either of the dilute acids because lead. Lead Carbonate With Nitric Acid Reaction.
From brainly.com
Solid lead(11) carbonate reacts with a dilute solution of nitric acid Lead Carbonate With Nitric Acid Reaction (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. Copper(ii) carbonate is an insoluble. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
Reaction of Nitric Acid with Metals, Chemistry Lecture Sabaq.pk YouTube Lead Carbonate With Nitric Acid Reaction lead and nitric acid reaction. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. , water and carbon dioxide are produced when acids react with carbonates. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. reactions of acids with carbonates. Here lead is oxidized to. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
of lead nitrate ,Thermal reaction YouTube Lead Carbonate With Nitric Acid Reaction Copper(ii) carbonate is an insoluble green powder. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. the reaction between copper(ii) carbonate and dilute sulfuric acid. , water and carbon dioxide are produced when acids react with carbonates. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Here lead. Lead Carbonate With Nitric Acid Reaction.
From brainly.in
Lead nitrate reacts with sulphuric acid to form white precipitate of Lead Carbonate With Nitric Acid Reaction reactions of acids with carbonates. Here lead is oxidized to its +2. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. lead has no noticeable reaction with either of the dilute acids because lead (ii). Lead Carbonate With Nitric Acid Reaction.
From www.slideshare.net
Acids And Bases Lead Carbonate With Nitric Acid Reaction experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. lead and nitric acid reaction. , water and carbon dioxide are produced when acids react with carbonates. Here lead is oxidized to its. Lead Carbonate With Nitric Acid Reaction.
From www.slideshare.net
Reaction types Lead Carbonate With Nitric Acid Reaction Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. , water and carbon dioxide are produced when acids react with carbonates. Here lead is oxidized to its +2. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. Copper(ii) carbonate is an insoluble green powder.. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for AgNO3 + FeCl3 = Fe(NO3)3 + AgCl Lead Carbonate With Nitric Acid Reaction Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. Here lead is oxidized to its +2. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. reactions of acids with carbonates.. Lead Carbonate With Nitric Acid Reaction.
From dxolebnih.blob.core.windows.net
Lead Carbonate Complexes at Justin Diaz blog Lead Carbonate With Nitric Acid Reaction Here lead is oxidized to its +2. lead has no noticeable reaction with either of the dilute acids because lead (ii) chloride and lead (ii) sulfate are insoluble in water. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. reactions of acids with carbonates. experiments. Lead Carbonate With Nitric Acid Reaction.
From www.chegg.com
Solved 3, lead(II) nitrate + potassium sulfate ? (NOs )2 4 Lead Carbonate With Nitric Acid Reaction reactions of acids with carbonates. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. lead and nitric acid reaction. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. Here. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
Reaction of Nitric Acid With Some Reducing Agents, Chemistry Lecture Lead Carbonate With Nitric Acid Reaction the reaction between copper(ii) carbonate and dilute sulfuric acid. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. Copper(ii) carbonate is an insoluble green powder. reactions of acids with carbonates. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. lead and nitric acid reaction.. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
AS/A Level ChemistryReaction of Lead Oxide and Nitric Acid. Calculate Lead Carbonate With Nitric Acid Reaction , water and carbon dioxide are produced when acids react with carbonates. reactions of acids with carbonates. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. the reaction between copper(ii) carbonate and dilute sulfuric acid. lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Copper(ii) carbonate is. Lead Carbonate With Nitric Acid Reaction.
From www.showme.com
Ashok (sodium carbonate and nitric acid) total ionic equation Science Lead Carbonate With Nitric Acid Reaction Copper(ii) carbonate is an insoluble green powder. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. the reaction between copper(ii) carbonate and dilute sulfuric acid. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. Here lead is oxidized to its +2. reactions of. Lead Carbonate With Nitric Acid Reaction.
From www.toppr.com
Write the balanced chemical equation the following reactions & identify Lead Carbonate With Nitric Acid Reaction Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. lead and nitric acid reaction. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. reactions of acids with carbonates. Copper(ii) carbonate is an insoluble green powder. the reaction between copper(ii) carbonate and dilute. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
Lead carbonate with Nitric acid YouTube Lead Carbonate With Nitric Acid Reaction Here lead is oxidized to its +2. lead has no noticeable reaction with either of the dilute acids because lead (ii) chloride and lead (ii) sulfate are insoluble in water. lead and nitric acid reaction. reactions of acids with carbonates. , water and carbon dioxide are produced when acids react with carbonates. Copper(ii) carbonate is an insoluble. Lead Carbonate With Nitric Acid Reaction.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Lead Carbonate With Nitric Acid Reaction Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii) carbonate with. the reaction between copper(ii). Lead Carbonate With Nitric Acid Reaction.
From www.fishersci.com
Lead(II) carbonate, ACS reagent, ACROS Organics Fisher Scientific Lead Carbonate With Nitric Acid Reaction lead and nitric acid reaction. , water and carbon dioxide are produced when acids react with carbonates. when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. experiments in. Lead Carbonate With Nitric Acid Reaction.
From www.youtube.com
reaction of lead nitrate class 10 of lead Lead Carbonate With Nitric Acid Reaction lead and nitric acid reaction. experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no 2 and water. Here lead is oxidized to its +2. (b) a solution of lead(ii) nitrate can be made by reacting solid lead(ii). Lead Carbonate With Nitric Acid Reaction.
From www.showme.com
ShowMe sodium carbonate and nitric acid Lead Carbonate With Nitric Acid Reaction when acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon. lead has no noticeable reaction with either of the dilute acids because lead (ii) chloride and lead (ii) sulfate are insoluble in water. Lead(pb) and dilute nitric acid react to form lead nitrate ( pb(no 3) 2), no. Lead Carbonate With Nitric Acid Reaction.
From www.slideshare.net
Precipitation react2 Lead Carbonate With Nitric Acid Reaction Copper(ii) carbonate is an insoluble green powder. lead has no noticeable reaction with either of the dilute acids because lead (ii) chloride and lead (ii) sulfate are insoluble in water. , water and carbon dioxide are produced when acids react with carbonates. Here lead is oxidized to its +2. (b) a solution of lead(ii) nitrate can be made. Lead Carbonate With Nitric Acid Reaction.