Units For Rate Constant For First Order Reaction . the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. For a zero order reaction, the rate. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1.
from www.brainkart.com
in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. For a zero order reaction, the rate. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time).
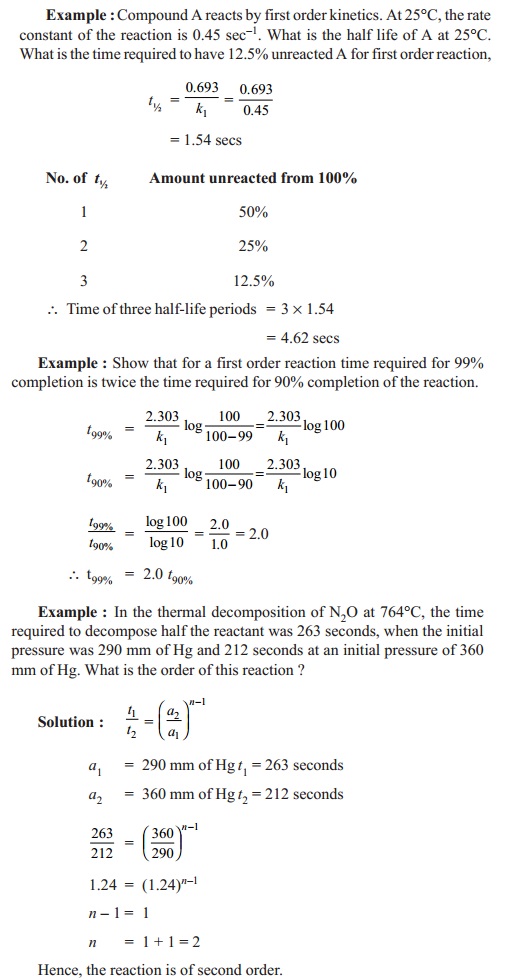
Rate equation for first order reactions
Units For Rate Constant For First Order Reaction more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. For a zero order reaction, the rate.
From www.brainkart.com
Rate equation for first order reactions Units For Rate Constant For First Order Reaction more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). For a zero order reaction, the rate. in general, for a reaction with order a + b, the units. Units For Rate Constant For First Order Reaction.
From www.chegg.com
Solved What is the rate constant of a firstorder reaction Units For Rate Constant For First Order Reaction more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. for the purposes of rate equations and orders of reaction, the rate of a reaction is. Units For Rate Constant For First Order Reaction.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5409963 Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. For a zero order reaction, the rate. in general, for a reaction with. Units For Rate Constant For First Order Reaction.
From mavink.com
First Order Reaction Units Units For Rate Constant For First Order Reaction the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). For a zero order reaction, the rate. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. in general, for a reaction with order a + b, the units. Units For Rate Constant For First Order Reaction.
From www.slideshare.net
Lect w2 152 rate laws_alg Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. For a zero order reaction, the rate. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. in general, for a reaction with. Units For Rate Constant For First Order Reaction.
From www.brainkart.com
Rate equation for first order reactions Units For Rate Constant For First Order Reaction For a zero order reaction, the rate. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. for the purposes of rate equations and orders of. Units For Rate Constant For First Order Reaction.
From byjus.com
What is the unit of rate constant for first order reaction Units For Rate Constant For First Order Reaction For a zero order reaction, the rate. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. the rate is the reaction rate. Units For Rate Constant For First Order Reaction.
From dxoaraavy.blob.core.windows.net
Rate Constant For First Order Reaction Is 2.303 at Iris Olson blog Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. the rate is the reaction rate. Units For Rate Constant For First Order Reaction.
From kelvinklein.weebly.com
DVHS Chemistry club Lecture Notes Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). For a zero order reaction, the rate. more generally. Units For Rate Constant For First Order Reaction.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6090305 Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. For a zero order reaction, the rate. the rate is the reaction rate. Units For Rate Constant For First Order Reaction.
From dxotqwilz.blob.core.windows.net
Formula Of Rate Constant For First Order Reaction at John Kingston blog Units For Rate Constant For First Order Reaction For a zero order reaction, the rate. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate. Units For Rate Constant For First Order Reaction.
From www.researchgate.net
Firstorder reaction rate constants (k obs ) and initial reaction rates Units For Rate Constant For First Order Reaction in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. For a zero order reaction, the rate.. Units For Rate Constant For First Order Reaction.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Units For Rate Constant For First Order Reaction For a zero order reaction, the rate. for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1.. Units For Rate Constant For First Order Reaction.
From dxoaraavy.blob.core.windows.net
Rate Constant For First Order Reaction Is 2.303 at Iris Olson blog Units For Rate Constant For First Order Reaction more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. For a zero order reaction, the rate. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). in general, for a reaction with order a + b, the units. Units For Rate Constant For First Order Reaction.
From www.researchgate.net
How to calculate rate constant for first order reaction? ResearchGate Units For Rate Constant For First Order Reaction in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate equations and orders of reaction, the rate. Units For Rate Constant For First Order Reaction.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions Units For Rate Constant For First Order Reaction in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). more generally speaking, the units for the rate constant for a reaction. Units For Rate Constant For First Order Reaction.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation, free download ID5609537 Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). in general, for a reaction with order a +. Units For Rate Constant For First Order Reaction.
From www.youtube.com
Quickvideo Calculating rate constant from a firstorder reaction Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. For a zero order reaction, the rate. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. in general, for a reaction with. Units For Rate Constant For First Order Reaction.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2012 PowerPoint Presentation, free Units For Rate Constant For First Order Reaction more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. the rate is the reaction rate (in units of molar/time) and k is the reaction rate. Units For Rate Constant For First Order Reaction.
From askfilo.com
Units of rate constant for a first order reaction. Filo Units For Rate Constant For First Order Reaction the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. For a zero order reaction, the rate. in general, for a reaction with order a + b, the units. Units For Rate Constant For First Order Reaction.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. in general, for a reaction with order a + b, the units of. Units For Rate Constant For First Order Reaction.
From byjus.com
What is the unit of rate for a first order reaction? Units For Rate Constant For First Order Reaction in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate equations and orders of reaction, the rate. Units For Rate Constant For First Order Reaction.
From dxorphpoy.blob.core.windows.net
The Rate Constant For A First Order Reaction At 300 at Ricky Williams blog Units For Rate Constant For First Order Reaction For a zero order reaction, the rate. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. more generally speaking, the units. Units For Rate Constant For First Order Reaction.
From www.toppr.com
Units of rate constant of a first order reaction is Units For Rate Constant For First Order Reaction in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. more generally speaking, the units for. Units For Rate Constant For First Order Reaction.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 Units For Rate Constant For First Order Reaction For a zero order reaction, the rate. for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1.. Units For Rate Constant For First Order Reaction.
From dxotqwilz.blob.core.windows.net
Formula Of Rate Constant For First Order Reaction at John Kingston blog Units For Rate Constant For First Order Reaction for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. more generally speaking, the units for. Units For Rate Constant For First Order Reaction.
From www.numerade.com
What are the units of the rate constant for (a) a firstorder reaction Units For Rate Constant For First Order Reaction in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. For a zero order reaction, the rate. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate. Units For Rate Constant For First Order Reaction.
From dxosqvjcq.blob.core.windows.net
Rate Constant For A First Order Reaction Is 2.303 at Joann Hacker blog Units For Rate Constant For First Order Reaction For a zero order reaction, the rate. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. in general,. Units For Rate Constant For First Order Reaction.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Units For Rate Constant For First Order Reaction in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. for the purposes of rate equations and orders of reaction, the rate of a reaction is. Units For Rate Constant For First Order Reaction.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Units For Rate Constant For First Order Reaction in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate equations and orders of reaction, the rate. Units For Rate Constant For First Order Reaction.
From www.youtube.com
The rate constant of a first order reaction is `3 xx 10^(6)" per sec Units For Rate Constant For First Order Reaction For a zero order reaction, the rate. for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1.. Units For Rate Constant For First Order Reaction.
From www.youtube.com
The rate constant for a first order reaction six times when the Units For Rate Constant For First Order Reaction more generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are. the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). in general, for a reaction with order a + b, the units of the rate constant are mol 1−. Units For Rate Constant For First Order Reaction.
From dxoaraavy.blob.core.windows.net
Rate Constant For First Order Reaction Is 2.303 at Iris Olson blog Units For Rate Constant For First Order Reaction the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). in general, for a reaction with order a + b, the units of the rate constant are mol 1− (m+n) ·l (m+n)−1 ·s −1. For a zero order reaction, the rate. for the purposes of rate. Units For Rate Constant For First Order Reaction.
From www.youtube.com
Units of rate constant calculate rate constant units units of first Units For Rate Constant For First Order Reaction the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. For a zero order reaction, the rate. more generally. Units For Rate Constant For First Order Reaction.
From www.brainkart.com
Rate equation for first order reactions Units For Rate Constant For First Order Reaction the rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). for the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. For a zero order reaction, the rate. more generally. Units For Rate Constant For First Order Reaction.