Labelling Of Medical Devices Eu . This document aims to provide guidance on different aspects related to standards in the medical. Medical devices are products or equipment intended for a medical purpose. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. Union can give binding interpretations of union law. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. In the european union (eu) they must undergo a conformity assessment to.
from gbu-taganskij.ru
This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. In the european union (eu) they must undergo a conformity assessment to. Medical devices are products or equipment intended for a medical purpose. This document aims to provide guidance on different aspects related to standards in the medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Union can give binding interpretations of union law.
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF
Labelling Of Medical Devices Eu This document aims to provide guidance on different aspects related to standards in the medical. Union can give binding interpretations of union law. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. This document aims to provide guidance on different aspects related to standards in the medical. In the european union (eu) they must undergo a conformity assessment to. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Medical devices are products or equipment intended for a medical purpose. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions.
From omcmedical.com
EU Classification of Medical Devices OMC Medical Labelling Of Medical Devices Eu This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. Medical devices are products or equipment intended for a medical purpose. This document aims to provide guidance on different aspects related to standards in the medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017. Labelling Of Medical Devices Eu.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Labelling Of Medical Devices Eu In the european union (eu) they must undergo a conformity assessment to. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. This document aims to provide guidance on different aspects related to standards in the medical. This guide covers labelling requirements for medical devices in the eu, such. Labelling Of Medical Devices Eu.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Of Medical Devices Eu Medical devices are products or equipment intended for a medical purpose. This document aims to provide guidance on different aspects related to standards in the medical. Union can give binding interpretations of union law. In the european union (eu) they must undergo a conformity assessment to. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if. Labelling Of Medical Devices Eu.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Labelling Of Medical Devices Eu These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity assessment to. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions.. Labelling Of Medical Devices Eu.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labelling Of Medical Devices Eu This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. In the european union (eu) they. Labelling Of Medical Devices Eu.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Labelling Of Medical Devices Eu Medical devices are products or equipment intended for a medical purpose. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. In the european union (eu) they must undergo a conformity assessment to. This document aims to provide guidance on different aspects related to standards in the medical. Regulation (eu) 2017/745 of. Labelling Of Medical Devices Eu.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Labelling Of Medical Devices Eu In the european union (eu) they must undergo a conformity assessment to. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: This guide covers labelling requirements for medical devices in the. Labelling Of Medical Devices Eu.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Labelling Of Medical Devices Eu Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Union can give binding interpretations of union law. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly. Labelling Of Medical Devices Eu.
From www.opal-labelmanagement.com
Labeling of medical devices according to EU MDR and UDI Labelling Of Medical Devices Eu These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. Medical devices. Labelling Of Medical Devices Eu.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Labelling Of Medical Devices Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Union can give binding interpretations of union law. Medical devices are products or equipment intended for a medical purpose. In the european. Labelling Of Medical Devices Eu.
From mavink.com
Medical Device Labeling Symbols Labelling Of Medical Devices Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. In the european union (eu) they must undergo a conformity assessment to. Medical devices are products or equipment intended for a. Labelling Of Medical Devices Eu.
From www.afpharmaservice.com
Medical Device Labelling Requirements Labelling Of Medical Devices Eu This document aims to provide guidance on different aspects related to standards in the medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Union can give binding interpretations of union. Labelling Of Medical Devices Eu.
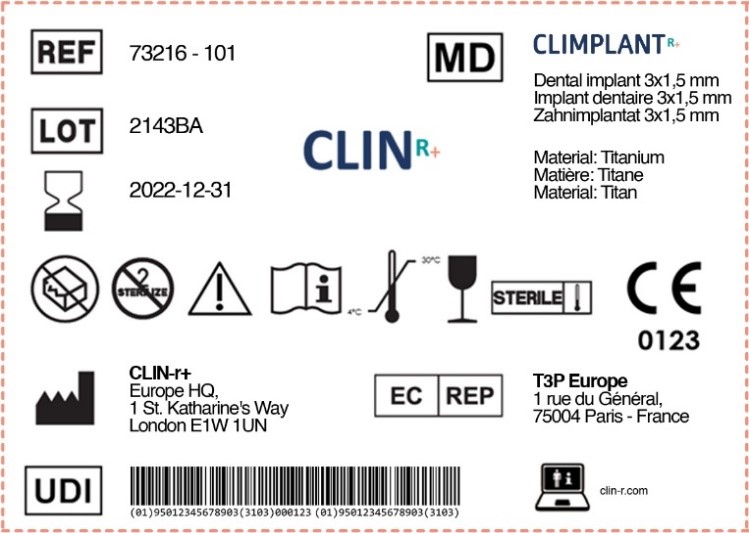
From clin-r.com
Labels for Medical Devices Clin R Labelling Of Medical Devices Eu Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Union can give binding interpretations of union law. This document aims to provide guidance on different aspects related to standards in the medical. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a. Labelling Of Medical Devices Eu.
From www.csoftintl.com
THE EU MDR LABELLING JOURNEY BEST PRACTICES FOR NAVIGATING THE LATEST Labelling Of Medical Devices Eu Union can give binding interpretations of union law. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017. Labelling Of Medical Devices Eu.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labelling Of Medical Devices Eu Medical devices are products or equipment intended for a medical purpose. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: In the european union (eu) they must undergo a conformity assessment to. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. This document. Labelling Of Medical Devices Eu.
From www.qualitymeddev.com
ISO 152232020 Update of for Symbols to be used with Medical Devices Labelling Of Medical Devices Eu Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. In the european union (eu) they. Labelling Of Medical Devices Eu.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Labelling Of Medical Devices Eu This document aims to provide guidance on different aspects related to standards in the medical. Union can give binding interpretations of union law. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament. Labelling Of Medical Devices Eu.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Labelling Of Medical Devices Eu These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Medical devices are products or equipment intended for a medical purpose. Union can give binding interpretations of union law. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. This document aims to. Labelling Of Medical Devices Eu.
From www.meddeviceonline.com
Infographic Medical Device Label Before And After EU MDR 10 Sticking Points Labelling Of Medical Devices Eu This document aims to provide guidance on different aspects related to standards in the medical. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. In the european union (eu) they must undergo a conformity assessment to. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april. Labelling Of Medical Devices Eu.
From www.tailoredlabel.com
Medical Device Regulation The Impact on Medical Device Labeling TLP Labelling Of Medical Devices Eu Union can give binding interpretations of union law. In the european union (eu) they must undergo a conformity assessment to. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. This guide covers labelling requirements for medical devices. Labelling Of Medical Devices Eu.
From www.mavenrs.uk
Medical Devices Labeling Checklist for EU MDR Compliance Maven Labelling Of Medical Devices Eu In the european union (eu) they must undergo a conformity assessment to. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Regulation (eu) 2017/745 of the european parliament and of the council of. Labelling Of Medical Devices Eu.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Of Medical Devices Eu These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. This document aims to provide guidance on different aspects related to standards in the medical. Union can give binding interpretations of union law. Medical devices are products or equipment intended for a medical purpose. This guide covers labelling requirements for medical devices. Labelling Of Medical Devices Eu.
From loeolkifl.blob.core.windows.net
What Is A Medical Device Eu at Jonathan Eady blog Labelling Of Medical Devices Eu Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: In the european union (eu) they must undergo a conformity assessment to. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. This document aims to provide guidance on different aspects related to standards in. Labelling Of Medical Devices Eu.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Labelling Of Medical Devices Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. This document aims to provide guidance on different aspects related to standards in the medical. Medical devices are products or equipment intended for a medical purpose. Union can give binding interpretations of union law. These tables aim to help. Labelling Of Medical Devices Eu.
From easymedicaldevice.com
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR) Labelling Of Medical Devices Eu This document aims to provide guidance on different aspects related to standards in the medical. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity assessment to. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Union can give binding. Labelling Of Medical Devices Eu.
From loeolkifl.blob.core.windows.net
What Is A Medical Device Eu at Jonathan Eady blog Labelling Of Medical Devices Eu Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity assessment to. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. This document aims to provide guidance on different aspects related to standards in the medical. This guide covers labelling. Labelling Of Medical Devices Eu.
From clin-r.com
Labels for Medical Devices Clin R Labelling Of Medical Devices Eu These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Union can give binding interpretations of union law. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: In the european union (eu) they must undergo a conformity assessment to. This guide covers labelling requirements. Labelling Of Medical Devices Eu.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Labelling Of Medical Devices Eu This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. This document aims to provide guidance on different aspects related to standards in the medical. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity assessment to. Union can give. Labelling Of Medical Devices Eu.
From exyrvtbcz.blob.core.windows.net
Symbols In Medical Device Labeling at Jillian Bundy blog Labelling Of Medical Devices Eu Medical devices are products or equipment intended for a medical purpose. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. In the european union (eu) they must undergo a conformity assessment. Labelling Of Medical Devices Eu.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labelling Of Medical Devices Eu Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. In the european union (eu) they must undergo a conformity assessment to. This document aims to provide guidance on different aspects related. Labelling Of Medical Devices Eu.
From coastlabel.com
Medical Device Labeling Medical Equipment Labels Coast Label Labelling Of Medical Devices Eu This document aims to provide guidance on different aspects related to standards in the medical. This guide covers labelling requirements for medical devices in the eu, such as ce marking, traceability information, and instructions. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: Union can give binding interpretations of union law. In. Labelling Of Medical Devices Eu.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Of Medical Devices Eu In the european union (eu) they must undergo a conformity assessment to. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Labelling Of Medical Devices Eu.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Of Medical Devices Eu Union can give binding interpretations of union law. This document aims to provide guidance on different aspects related to standards in the medical. Information for users (labeling/ifu) • labeling requirements (23.2) • label must have indication if the device incorporates: These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly small and. This guide. Labelling Of Medical Devices Eu.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labelling Of Medical Devices Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity assessment to. These tables aim to help manufacturers of medical devices and in vitro diagnostic medical devices, particularly. Labelling Of Medical Devices Eu.
From www.microscan.com
Label Compliance and the New European Medical Device Regulations Labelling Of Medical Devices Eu In the european union (eu) they must undergo a conformity assessment to. This document aims to provide guidance on different aspects related to standards in the medical. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive 2001/83/ec,. Union. Labelling Of Medical Devices Eu.