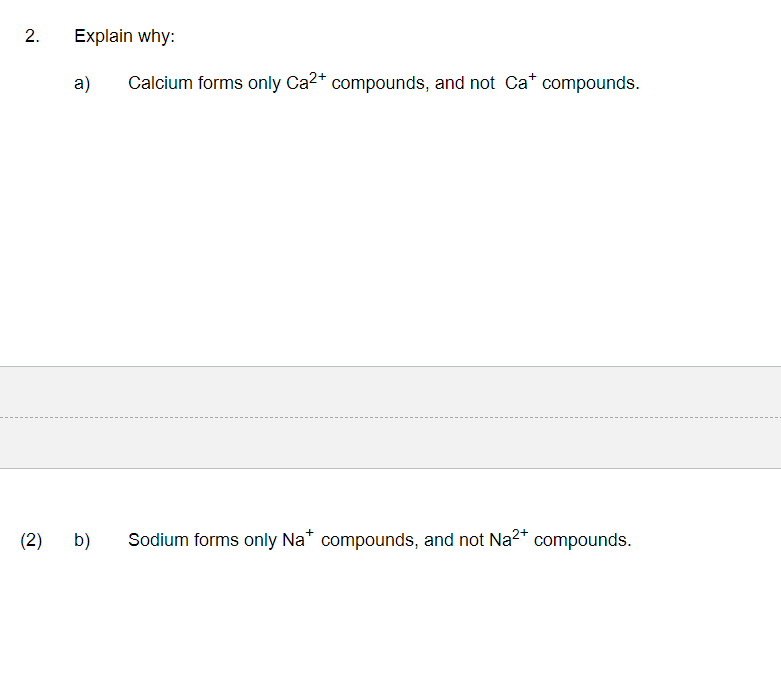
Why Is Ca Bigger Than Ca2 . updated on november 05, 2019. for a) the neutral ca atom has a larger radius than the ca2+ ion. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). rather, the ca ion is in a net +2 state. Think of it this way: This is because the loss of electrons reduces the shielding effect between. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. When an atom loses an electron to. And to indicate this state, we first indicate what's most important (the. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. 1) count the electrons (all of them) in each atom or ion. ions may be larger or smaller than the neutral atom, depending on the ion's electric charge.
from www.chegg.com
The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. Think of it this way: And to indicate this state, we first indicate what's most important (the. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. updated on november 05, 2019. rather, the ca ion is in a net +2 state. for a) the neutral ca atom has a larger radius than the ca2+ ion. When an atom loses an electron to. ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca).
Solved Explain why a) Calcium forms only Ca2+ compounds,
Why Is Ca Bigger Than Ca2 And to indicate this state, we first indicate what's most important (the. And to indicate this state, we first indicate what's most important (the. 1) count the electrons (all of them) in each atom or ion. When an atom loses an electron to. updated on november 05, 2019. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). This is because the loss of electrons reduces the shielding effect between. rather, the ca ion is in a net +2 state. Think of it this way: ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. for a) the neutral ca atom has a larger radius than the ca2+ ion. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius.
From chemistry.stackexchange.com
atomic radius Why does Mg2+ have the ionic radii bigger than Ca2 Why Is Ca Bigger Than Ca2 for a) the neutral ca atom has a larger radius than the ca2+ ion. ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. Think of it this way: The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. And to indicate this state, we. Why Is Ca Bigger Than Ca2.
From www.chegg.com
Solved Explain why a) Calcium forms only Ca2+ compounds, Why Is Ca Bigger Than Ca2 1) count the electrons (all of them) in each atom or ion. And to indicate this state, we first indicate what's most important (the. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). rather, the ca ion is in a net +2 state. This is because the loss of electrons reduces the shielding effect between.. Why Is Ca Bigger Than Ca2.
From www.numerade.com
SOLVED K+ is larger than Ca2+, which is best explained because K+ Why Is Ca Bigger Than Ca2 When an atom loses an electron to. Think of it this way: 1) count the electrons (all of them) in each atom or ion. And to indicate this state, we first indicate what's most important (the. ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. rather, the ca ion is in. Why Is Ca Bigger Than Ca2.
From slideplayer.com
AP Chemistry Periodicity. ppt download Why Is Ca Bigger Than Ca2 updated on november 05, 2019. ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. for a) the neutral ca atom has a larger radius than the ca2+ ion. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. the calcium ion (ca2+). Why Is Ca Bigger Than Ca2.
From mavink.com
Calcium Orbital Diagram Why Is Ca Bigger Than Ca2 1) count the electrons (all of them) in each atom or ion. for a) the neutral ca atom has a larger radius than the ca2+ ion. This is because the loss of electrons reduces the shielding effect between. updated on november 05, 2019. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). rather,. Why Is Ca Bigger Than Ca2.
From slideplayer.com
Dendritic Spine Geometry Functional Implication and Regulation ppt Why Is Ca Bigger Than Ca2 And to indicate this state, we first indicate what's most important (the. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). Think of it this way: The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. updated on november 05, 2019. for a) the neutral ca atom. Why Is Ca Bigger Than Ca2.
From slideplayer.com
AP Chemistry Due Next Class Read up on our lab next class ppt download Why Is Ca Bigger Than Ca2 When an atom loses an electron to. This is because the loss of electrons reduces the shielding effect between. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. Think of it this way: for a) the neutral ca atom has a larger radius than the. Why Is Ca Bigger Than Ca2.
From www.researchgate.net
Mean first passage time of Ca2+ binding to CaM. (A) The mean transition Why Is Ca Bigger Than Ca2 And to indicate this state, we first indicate what's most important (the. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). Think of it this way: 1) count the electrons (all of them) in each atom or ion. rather, the ca ion is in a net +2 state. for a) the neutral ca atom. Why Is Ca Bigger Than Ca2.
From freyabartlett.z13.web.core.windows.net
Size Of Atoms Chart Why Is Ca Bigger Than Ca2 This is because the loss of electrons reduces the shielding effect between. updated on november 05, 2019. Think of it this way: rather, the ca ion is in a net +2 state. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having. Why Is Ca Bigger Than Ca2.
From schematicwawdr5.z21.web.core.windows.net
Orbital Diagram For Ca2+ Why Is Ca Bigger Than Ca2 When an atom loses an electron to. This is because the loss of electrons reduces the shielding effect between. rather, the ca ion is in a net +2 state. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is. Why Is Ca Bigger Than Ca2.
From www.slideshare.net
Cl has larger radius than Ca2+SolutionCl has larger radius t.pdf Why Is Ca Bigger Than Ca2 And to indicate this state, we first indicate what's most important (the. When an atom loses an electron to. This is because the loss of electrons reduces the shielding effect between. 1) count the electrons (all of them) in each atom or ion. ions may be larger or smaller than the neutral atom, depending on the ion's electric charge.. Why Is Ca Bigger Than Ca2.
From www.researchgate.net
PUAEC and NPUAEC show unique [Ca2+]i responses to ATP stimulation Why Is Ca Bigger Than Ca2 a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. rather, the ca ion is in a net +2 state. 1) count the electrons (all of them) in each atom or ion. for a) the neutral ca atom has a larger radius than the ca2+. Why Is Ca Bigger Than Ca2.
From slideplayer.com
Trends in Periodic Table ppt download Why Is Ca Bigger Than Ca2 a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. Think of it this way: When an atom loses an electron to. rather, the ca ion is in a. Why Is Ca Bigger Than Ca2.
From www.numerade.com
SOLVED Briefly explain why you can use this experiment's procedure to Why Is Ca Bigger Than Ca2 the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). 1) count the electrons (all of them) in each atom or ion. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. rather, the ca ion is in a net +2 state. a comparison of ionic radii with. Why Is Ca Bigger Than Ca2.
From travelingbase.com
Is California Bigger Than England? Traveling Base Why Is Ca Bigger Than Ca2 When an atom loses an electron to. 1) count the electrons (all of them) in each atom or ion. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. This is because the loss. Why Is Ca Bigger Than Ca2.
From www.researchgate.net
Interleaved imaging of Ca2+ puffs in TIRF and in widefield modes. (A Why Is Ca Bigger Than Ca2 This is because the loss of electrons reduces the shielding effect between. Think of it this way: for a) the neutral ca atom has a larger radius than the ca2+ ion. updated on november 05, 2019. And to indicate this state, we first indicate what's most important (the. the calcium ion (ca2+) is smaller than the neutral. Why Is Ca Bigger Than Ca2.
From www.ahajournals.org
Inherited Dysfunction of Sarcoplasmic Reticulum Ca2+ Handling and Why Is Ca Bigger Than Ca2 The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). Think of it this way: This is because the loss of electrons reduces the shielding effect between. updated on november 05, 2019. rather, the ca ion is in. Why Is Ca Bigger Than Ca2.
From www.youtube.com
Which is larger? Ca or Ca 2+ (Calcium atom or Calcium ion) YouTube Why Is Ca Bigger Than Ca2 a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. When an atom loses an electron to. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). Think of it this way: updated on november 05, 2019. ions may be larger or. Why Is Ca Bigger Than Ca2.
From www.numerade.com
SOLVED Predict the larger ion in each of the following pairs. (a) F Why Is Ca Bigger Than Ca2 ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. for a) the neutral ca atom has a larger radius than the ca2+ ion. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. rather, the ca ion is in a net +2 state.. Why Is Ca Bigger Than Ca2.
From www.numerade.com
SOLVED Determine which is the larger species. Ca2+ Ca Determine which Why Is Ca Bigger Than Ca2 1) count the electrons (all of them) in each atom or ion. updated on november 05, 2019. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. for a) the neutral ca atom has a larger radius than the ca2+ ion. a comparison of ionic radii with atomic radii (figure. Why Is Ca Bigger Than Ca2.
From slideplayer.com
Unit 01 (Chp 6,7) Atoms and Periodic Properties ppt download Why Is Ca Bigger Than Ca2 The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). updated on november 05, 2019. for a) the neutral ca atom has a larger radius than the ca2+ ion. When an atom loses an electron to. 1) count. Why Is Ca Bigger Than Ca2.
From slideplayer.com
CARDIAC MUSCLE CONTRACTILE MECHANISM OF CARDIAC MUSCLE ppt download Why Is Ca Bigger Than Ca2 updated on november 05, 2019. And to indicate this state, we first indicate what's most important (the. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). The size of atoms of elements. Why Is Ca Bigger Than Ca2.
From slideplayer.com
Periodic Properties of the Elements ppt download Why Is Ca Bigger Than Ca2 This is because the loss of electrons reduces the shielding effect between. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. rather, the ca ion is in a. Why Is Ca Bigger Than Ca2.
From www.frontiersin.org
Frontiers Bound Ca2+ moves faster and farther from single open Why Is Ca Bigger Than Ca2 the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. 1) count the electrons (all of them) in each atom or ion. for a) the neutral ca atom has a larger radius than the ca2+ ion. a comparison. Why Is Ca Bigger Than Ca2.
From howtravelplan.com
Is California Bigger Than England A Comparison Of Size Population And Why Is Ca Bigger Than Ca2 The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. This is because the loss of electrons reduces the shielding effect between. When. Why Is Ca Bigger Than Ca2.
From mapsofeverything.wixsite.com
California bigger than Canada Why Is Ca Bigger Than Ca2 When an atom loses an electron to. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). Think of it this way: updated on november 05, 2019. ions may be larger or. Why Is Ca Bigger Than Ca2.
From www.numerade.com
SOLVED K+ is larger than Ca2+, which is best explained because K+ Why Is Ca Bigger Than Ca2 for a) the neutral ca atom has a larger radius than the ca2+ ion. Think of it this way: rather, the ca ion is in a net +2 state. When an atom loses an electron to. updated on november 05, 2019. And to indicate this state, we first indicate what's most important (the. the calcium ion. Why Is Ca Bigger Than Ca2.
From slideplayer.com
1st semester exam review ppt download Why Is Ca Bigger Than Ca2 This is because the loss of electrons reduces the shielding effect between. When an atom loses an electron to. rather, the ca ion is in a net +2 state. updated on november 05, 2019. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. 1) count the electrons (all of them). Why Is Ca Bigger Than Ca2.
From travelingbase.com
Is California Bigger Than Alaska? Traveling Base Why Is Ca Bigger Than Ca2 The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. This is because the loss of electrons reduces the shielding effect between. rather, the ca ion is in a net +2 state. 1) count the electrons (all of them) in each atom or ion. updated on november 05, 2019. When an. Why Is Ca Bigger Than Ca2.
From www.youtube.com
Calculate the number of `Cl^()` and `Ca^(2+)` ions in `222 g Why Is Ca Bigger Than Ca2 a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. Think of it this way: updated. Why Is Ca Bigger Than Ca2.
From www.chegg.com
Solved Why does the calcium ( Ca2+) move into the axon Why Is Ca Bigger Than Ca2 updated on november 05, 2019. Think of it this way: The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. This is because the loss of electrons reduces the shielding effect between. When an atom loses an electron to. 1) count the electrons (all of them) in each atom or ion. . Why Is Ca Bigger Than Ca2.
From slideplayer.com
Elemental Properties and Patterns ppt download Why Is Ca Bigger Than Ca2 1) count the electrons (all of them) in each atom or ion. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). for a) the neutral ca atom has a larger radius than the ca2+ ion. rather, the. Why Is Ca Bigger Than Ca2.
From www.pinterest.com
Ca 2+ Electron Configuration (Calcium Ion) Electron configuration Why Is Ca Bigger Than Ca2 a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. When an atom loses an electron to. for a) the neutral ca atom has a larger radius than the ca2+ ion. updated on november 05, 2019. The size of atoms of elements may be expressed. Why Is Ca Bigger Than Ca2.
From www.researchgate.net
Membrane potential responses of the cardiac muscle to CA1 (upper trace Why Is Ca Bigger Than Ca2 for a) the neutral ca atom has a larger radius than the ca2+ ion. 1) count the electrons (all of them) in each atom or ion. updated on november 05, 2019. When an atom loses an electron to. the calcium ion (ca2+) is smaller than the neutral calcium atom (ca). ions may be larger or smaller. Why Is Ca Bigger Than Ca2.
From slideplayer.com
Chemical Bonding part ppt download Why Is Ca Bigger Than Ca2 for a) the neutral ca atom has a larger radius than the ca2+ ion. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. When an atom loses an electron to. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its. Why Is Ca Bigger Than Ca2.