Medical Device Labeling Checklist . Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). adequate labeling for a medical device requires proper design and procurement of the labels and labeling. But what is exactly required? the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,.
from www.qualtechs.com
labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). But what is exactly required? learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,.
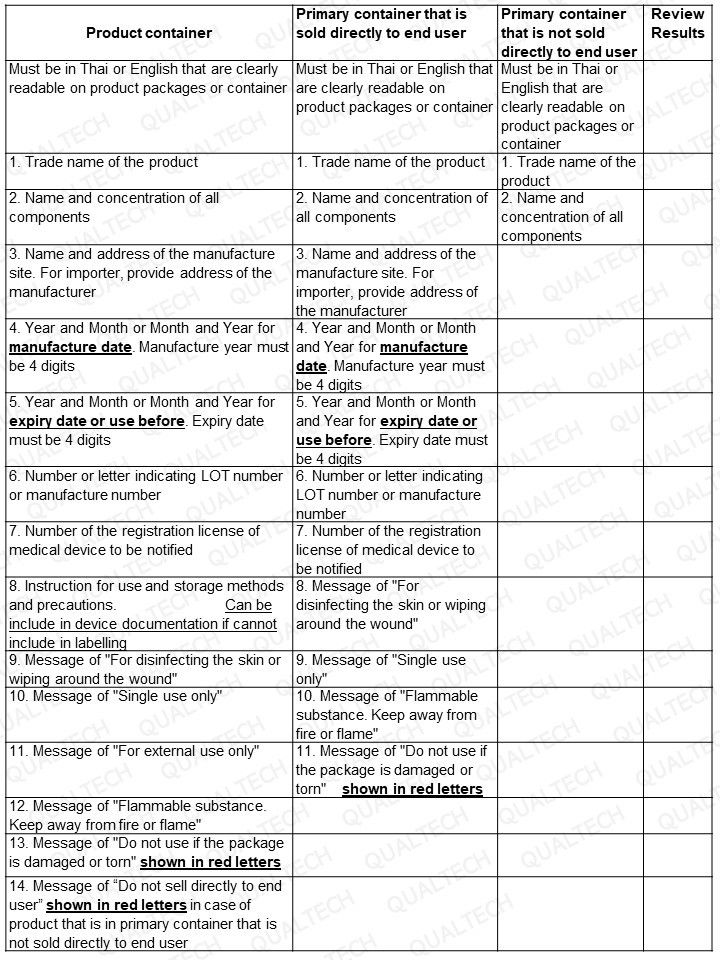
THAILAND Labelling Checklist of Cleaning Products Containing Alcohol
Medical Device Labeling Checklist labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: adequate labeling for a medical device requires proper design and procurement of the labels and labeling. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. But what is exactly required? learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. Discover the requirements mdr lists for medical device labelling and instructions for use (ifu).
From ambitiousmares.blogspot.com
35 Medical Device Label Labels Design Ideas 2020 Medical Device Labeling Checklist Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: But what is exactly required? learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,.. Medical Device Labeling Checklist.
From mavink.com
Medical Device Labeling Symbols Medical Device Labeling Checklist adequate labeling for a medical device requires proper design and procurement of the labels and labeling. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). labeling regulations pertaining to medical devices are found. Medical Device Labeling Checklist.
From medicaldeviceacademy.com
FDA medical device labeling regulations Archives Medical Device Academy Medical Device Labeling Checklist Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). adequate labeling for a medical device requires proper design and procurement of the labels and labeling. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the use of symbols on the. Medical Device Labeling Checklist.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Medical Device Labeling Checklist labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. But what is exactly required? the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: adequate labeling for a medical device requires proper design and procurement of. Medical Device Labeling Checklist.
From mungfali.com
Medical Device Labeling Symbols Medical Device Labeling Checklist the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. But what is exactly required? labeling regulations pertaining to medical devices are found in the following parts of. Medical Device Labeling Checklist.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Medical Device Labeling Checklist adequate labeling for a medical device requires proper design and procurement of the labels and labeling. learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: But what. Medical Device Labeling Checklist.
From www.mavenrs.uk
Medical Devices Labeling Checklist for EU MDR Compliance Maven Medical Device Labeling Checklist the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: adequate labeling for a medical device requires proper design and procurement of the labels and labeling. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. . Medical Device Labeling Checklist.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Medical Device Labeling Checklist the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: But what is exactly required? adequate labeling for a medical device requires proper design and procurement of the labels and labeling. learn the minimum labeling requirements for medical devices in the u.s., such as name and place of. Medical Device Labeling Checklist.
From ectcolllefi.cf
Iso 15223 1 2012 Medical Devices symbols to be Used with Medical device Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: But what is exactly required? Discover the requirements mdr lists for medical device labelling and instructions for use (ifu).. Medical Device Labeling Checklist.
From lsacademy.com
Labelling Requirements for Medical Devices LS Academy Medical Device Labeling Checklist the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Medical Device Labeling Checklist.
From www.greenlight.guru
Medical Device Labeling Definition & Requirements Medical Device Labeling Checklist adequate labeling for a medical device requires proper design and procurement of the labels and labeling. But what is exactly required? labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the use of symbols on the label as an alternative to written language is permitted. Medical Device Labeling Checklist.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Medical Device Labeling Checklist But what is exactly required? learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the use of symbols on the label as an alternative to. Medical Device Labeling Checklist.
From www.pdffiller.com
Fillable Online How to use a labeling checklist for medical devices Medical Device Labeling Checklist Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. But what is. Medical Device Labeling Checklist.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). adequate labeling for a medical device requires proper design and procurement of the labels and labeling. But what is exactly required? labeling regulations. Medical Device Labeling Checklist.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Labeling Checklist Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). But what is exactly required? learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation:. Medical Device Labeling Checklist.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Labeling Checklist But what is exactly required? adequate labeling for a medical device requires proper design and procurement of the labels and labeling. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the. Medical Device Labeling Checklist.
From aditi.du.ac.in
MDR Requirements For Device Labeling And Implant Card, 07/24/2023 Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. adequate labeling for a medical device requires proper design and procurement of the labels and labeling. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: But what. Medical Device Labeling Checklist.
From www.qualtechs.com
THAILAND Labelling Checklist of Cleaning Products Containing Alcohol Medical Device Labeling Checklist labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. adequate labeling for a medical device requires proper design and procurement of the labels and labeling. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: Discover. Medical Device Labeling Checklist.
From www.scilife.io
Labeling Requirements for Medical Devices Scilife Medical Device Labeling Checklist labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Discover. Medical Device Labeling Checklist.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Checklist the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: adequate labeling for a medical device requires proper design and procurement of the labels and labeling. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. . Medical Device Labeling Checklist.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Medical Device Labeling Checklist the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). adequate labeling for a medical device requires proper design and procurement of the labels and labeling. learn the minimum labeling requirements for medical devices. Medical Device Labeling Checklist.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. adequate labeling for a medical device requires proper design and procurement of the labels and labeling. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations.. Medical Device Labeling Checklist.
From dxolizkya.blob.core.windows.net
Medical Device Labelling Requirements at William Smith blog Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. But what is exactly required? adequate labeling for a medical device requires proper design and procurement of the labels and labeling. the use of symbols on the label as an alternative to written language is permitted in. Medical Device Labeling Checklist.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. adequate labeling. Medical Device Labeling Checklist.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Medical Device Labeling Checklist labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: But what is exactly. Medical Device Labeling Checklist.
From www.schlafenderhase.com
A Guide to Medical Device Labeling Requirements Schlafender Hase Medical Device Labeling Checklist But what is exactly required? Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). adequate labeling for a medical device requires proper design and procurement of the labels and labeling. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the. Medical Device Labeling Checklist.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Medical Device Labeling Checklist labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). But what is exactly required? adequate labeling for a medical device requires proper design and procurement of the labels and labeling. learn. Medical Device Labeling Checklist.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). But what is exactly required? labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of. Medical Device Labeling Checklist.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Checklist Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: labeling regulations pertaining to. Medical Device Labeling Checklist.
From www.youtube.com
How to use a labeling checklist for medical devices YouTube Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). But what is exactly required?. Medical Device Labeling Checklist.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Checklist Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: But what is exactly required? learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,.. Medical Device Labeling Checklist.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Checklist the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. But what is exactly required? adequate labeling for a medical device requires proper design and procurement of the. Medical Device Labeling Checklist.
From mdlaw.eu
MDR Checklist Labelling & IFU Requirements · MDlaw Information Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. But what is exactly required? Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). adequate labeling for a medical device requires proper design and procurement of the labels and labeling. labeling regulations. Medical Device Labeling Checklist.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Labeling Checklist learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. But what is exactly required? Discover the requirements mdr lists for medical device labelling and instructions for use (ifu). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of. Medical Device Labeling Checklist.
From www.mavenrs.com
Medical Devices Labeling Checklist for EU MDR Compliance Maven Medical Device Labeling Checklist But what is exactly required? learn the minimum labeling requirements for medical devices in the u.s., such as name and place of business, intended use,. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: Discover the requirements mdr lists for medical device labelling and instructions for use (ifu).. Medical Device Labeling Checklist.