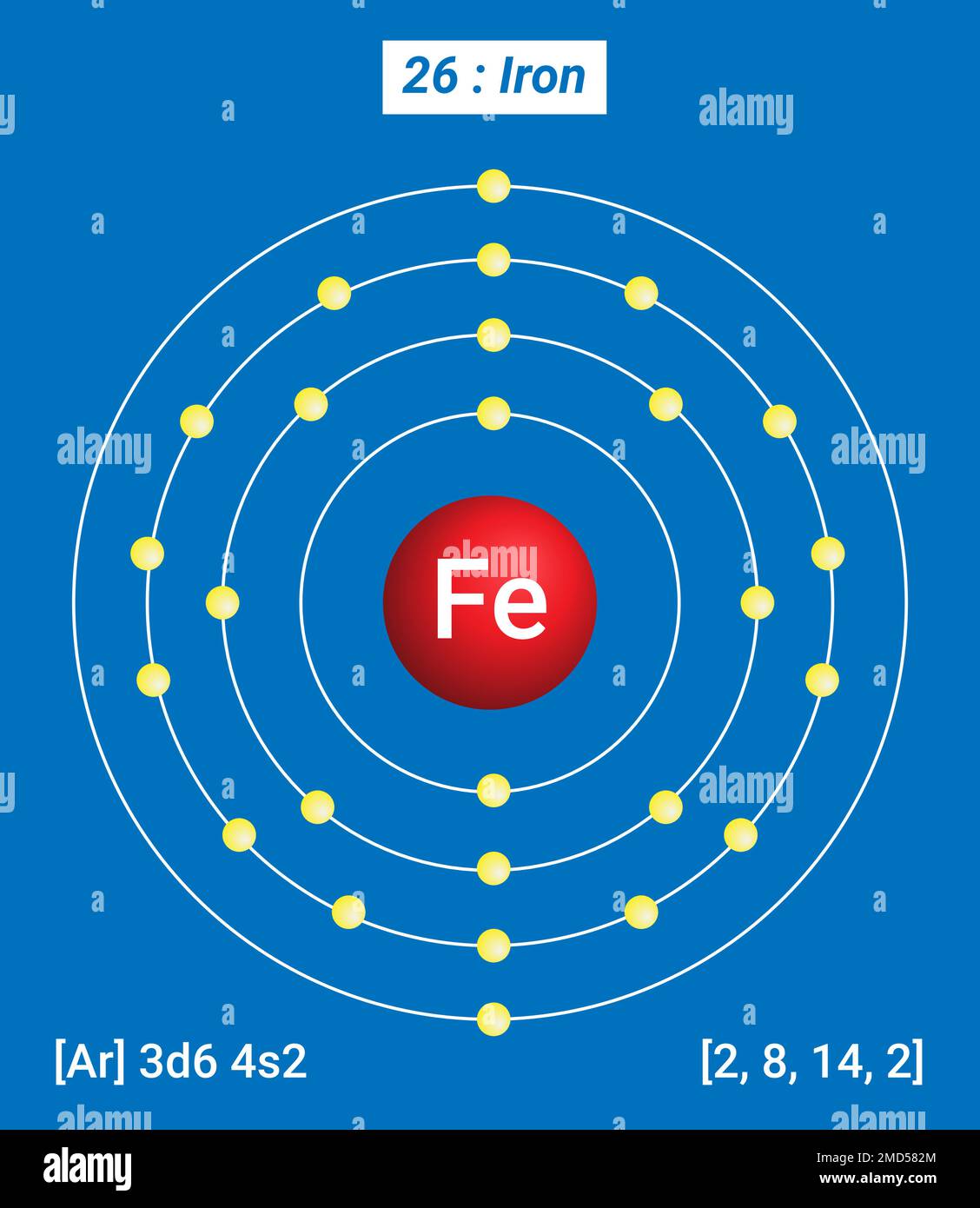
Iron Electrons Atom . Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The core of the earth is thought to be largely composed of iron with nickel and. Iron is the fourth most abundant element, by mass, in the earth’s crust. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Iron can form various oxidation states, the most. It’s a transition metal from group 8 of the periodic table’s first transition series. The electron configuration of iron is [ar] 3d6 4s2. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. This configuration reveals iron’s chemical behavior.
from www.alamy.com
Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Iron can form various oxidation states, the most. It’s a transition metal from group 8 of the periodic table’s first transition series. Iron is the fourth most abundant element, by mass, in the earth’s crust. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The core of the earth is thought to be largely composed of iron with nickel and. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#.
Fe Iron, Periodic Table of the Elements, Shell Structure of Iron
Iron Electrons Atom Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Iron is the fourth most abundant element, by mass, in the earth’s crust. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The electron configuration of iron is [ar] 3d6 4s2. It’s a transition metal from group 8 of the periodic table’s first transition series. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The core of the earth is thought to be largely composed of iron with nickel and. This configuration reveals iron’s chemical behavior. Iron can form various oxidation states, the most.
From www.animalia-life.club
Iron Atom Iron Electrons Atom The electron configuration of iron is [ar] 3d6 4s2. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This configuration reveals iron’s chemical behavior. The core of the earth is thought to be largely composed of iron with nickel and. Its core orbitals are the #1s#,. Iron Electrons Atom.
From rapidelectron.blogspot.com
Electron Configuration For An Atom Of Iron Rapid Electron Iron Electrons Atom This configuration reveals iron’s chemical behavior. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The electron configuration of iron is [ar] 3d6 4s2.. Iron Electrons Atom.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Electrons Atom The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron can form various oxidation states, the most. Iron. Iron Electrons Atom.
From dustinminlarson.blogspot.com
Write the Complete Electron Configuration for the Iron Atom Iron Electrons Atom This configuration reveals iron’s chemical behavior. Iron is the fourth most abundant element, by mass, in the earth’s crust. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. The chemical element iron has the atomic number 26 and. Iron Electrons Atom.
From www.alamy.com
Symbol and electron diagram for Iron illustration Stock Vector Image Iron Electrons Atom The chemical element iron has the atomic number 26 and the symbol fe (from latin: The core of the earth is thought to be largely composed of iron with nickel and. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Iron is a chemical element with. Iron Electrons Atom.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo Bigstock Iron Electrons Atom Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Iron is the fourth most abundant element, by mass, in the earth’s crust. The core of the earth is thought to be largely composed of iron with nickel and. This configuration reveals iron’s chemical behavior. Iron is a chemical element with. Iron Electrons Atom.
From www.alamy.com
Iron Molecule High Resolution Stock Photography and Images Alamy Iron Electrons Atom Iron is the fourth most abundant element, by mass, in the earth’s crust. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#.. Iron Electrons Atom.
From www.freepik.com
Premium Vector Iron atom bohr model Iron Electrons Atom The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This configuration reveals iron’s chemical behavior. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The core of the earth is thought to be largely composed of iron with nickel and.. Iron Electrons Atom.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Iron Electrons Atom Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The chemical element iron has the. Iron Electrons Atom.
From www.alamy.com
Iron (Fe). Diagram of the nuclear composition and electron Iron Electrons Atom The core of the earth is thought to be largely composed of iron with nickel and. Iron is the fourth most abundant element, by mass, in the earth’s crust. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Iron can form various oxidation states, the most.. Iron Electrons Atom.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Iron Electrons Atom This configuration reveals iron’s chemical behavior. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The electron configuration of iron is [ar] 3d6 4s2. Iron is a chemical element with atomic number 26. Iron Electrons Atom.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Electrons Atom The electron configuration of iron is [ar] 3d6 4s2. The core of the earth is thought to be largely composed of iron with nickel and. Iron is the fourth most abundant element, by mass, in the earth’s crust. It’s a transition metal from group 8 of the periodic table’s first transition series. Iron is on the fourth row of the. Iron Electrons Atom.
From www.animalia-life.club
Iron Atom Iron Electrons Atom Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. This configuration reveals iron’s chemical behavior. The. Iron Electrons Atom.
From www.bigstockphoto.com
3d Render Atom Structure Iron Image & Photo Bigstock Iron Electrons Atom The chemical element iron has the atomic number 26 and the symbol fe (from latin: Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. This configuration reveals iron’s chemical behavior. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number. Iron Electrons Atom.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Electrons Atom Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The core of the earth is thought to be largely composed of iron with nickel and. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Iron can. Iron Electrons Atom.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Electrons Atom The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. This configuration reveals iron’s chemical behavior.. Iron Electrons Atom.
From guidedehartfederalist.z21.web.core.windows.net
Iron Electron Dot Diagram Iron Electrons Atom Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. It’s a transition metal from group 8 of the periodic table’s first transition series. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Iron is the fourth most. Iron Electrons Atom.
From www.dreamstime.com
Atom of Iron with Detailed Core and Its 26 Electrons on Black Stock Iron Electrons Atom Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. It’s a transition metal from group 8 of the periodic table’s first transition series. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The electron configuration of iron is [ar] 3d6 4s2. Iron. Iron Electrons Atom.
From proper-cooking.info
Iron Atomic Structure Iron Electrons Atom The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. This configuration reveals iron’s chemical behavior. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The electron configuration. Iron Electrons Atom.
From www.nuclear-power.com
Iron Atomic Number Atomic Mass Density of Iron Iron Electrons Atom Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. This configuration reveals iron’s chemical behavior. The core of the earth is thought to be largely composed of iron with nickel and. Total number of protons in the nucleus is called the atomic number of the atom and is given the. Iron Electrons Atom.
From www.bigstockphoto.com
Iron Atom Image & Photo (Free Trial) Bigstock Iron Electrons Atom Iron is the fourth most abundant element, by mass, in the earth’s crust. The electron configuration of iron is [ar] 3d6 4s2. This configuration reveals iron’s chemical behavior. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic. Iron Electrons Atom.
From sciencenotes.org
Iron Atom Science Notes and Projects Iron Electrons Atom Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The core of the earth is thought to be largely composed of iron with nickel and. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. It’s a transition metal. Iron Electrons Atom.
From www.alamy.com
Fe Iron, Periodic Table of the Elements, Shell Structure of Iron Iron Electrons Atom The core of the earth is thought to be largely composed of iron with nickel and. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The electron configuration of iron is [ar]. Iron Electrons Atom.
From ar.inspiredpencil.com
Iron Atomic Structure Iron Electrons Atom Iron is the fourth most abundant element, by mass, in the earth’s crust. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron Electrons Atom.
From www.britannica.com
Iron Element, Occurrence, Uses, Properties, & Compounds Britannica Iron Electrons Atom The electron configuration of iron is [ar] 3d6 4s2. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The core of the earth is thought to be largely composed of iron with nickel. Iron Electrons Atom.
From www.alamy.com
Atom of Iron with detailed Core and its 26 Electrons with Atoms in Iron Electrons Atom Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This configuration reveals. Iron Electrons Atom.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electrons Atom It’s a transition metal from group 8 of the periodic table’s first transition series. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The core of the earth is thought to be largely composed of iron with nickel and. Iron is a chemical element with atomic number 26 which means there are 26 protons in its. Iron Electrons Atom.
From ar.inspiredpencil.com
Iron Atomic Structure Iron Electrons Atom Iron can form various oxidation states, the most. Iron is the fourth most abundant element, by mass, in the earth’s crust. This configuration reveals iron’s chemical behavior. It’s a transition metal from group 8 of the periodic table’s first transition series. The core of the earth is thought to be largely composed of iron with nickel and. The chemical element. Iron Electrons Atom.
From www.animalia-life.club
Iron Atom Iron Electrons Atom The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Iron can form various oxidation states, the most. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Iron is on the fourth row of the periodic table, sixth. Iron Electrons Atom.
From www.dreamstime.com
Atom of Iron with Detailed Core and Its 26 Electrons on Black Stock Iron Electrons Atom Iron can form various oxidation states, the most. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from group 8 of the periodic table’s first transition series. The core. Iron Electrons Atom.
From www.sciencephoto.com
Iron, atomic structure Stock Image C013/1539 Science Photo Library Iron Electrons Atom The core of the earth is thought to be largely composed of iron with nickel and. Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron can form various oxidation states, the most. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. This configuration reveals iron’s chemical behavior. Iron is on the. Iron Electrons Atom.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Electrons Atom This configuration reveals iron’s chemical behavior. Iron can form various oxidation states, the most. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Iron is a chemical element with. Iron Electrons Atom.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Electrons Atom Iron can form various oxidation states, the most. Iron is the fourth most abundant element, by mass, in the earth’s crust. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. This configuration reveals iron’s chemical behavior. Iron is a chemical element with atomic number 26 which means there are 26. Iron Electrons Atom.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Iron Electrons Atom The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from group 8 of the periodic table’s first transition series. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. This configuration reveals iron’s chemical behavior. Iron can form various oxidation states,. Iron Electrons Atom.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Electrons Atom The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Iron can form various oxidation states, the most. The core of the earth is thought to be largely composed of iron with nickel and. The chemical element iron has the atomic number 26 and the symbol fe. Iron Electrons Atom.