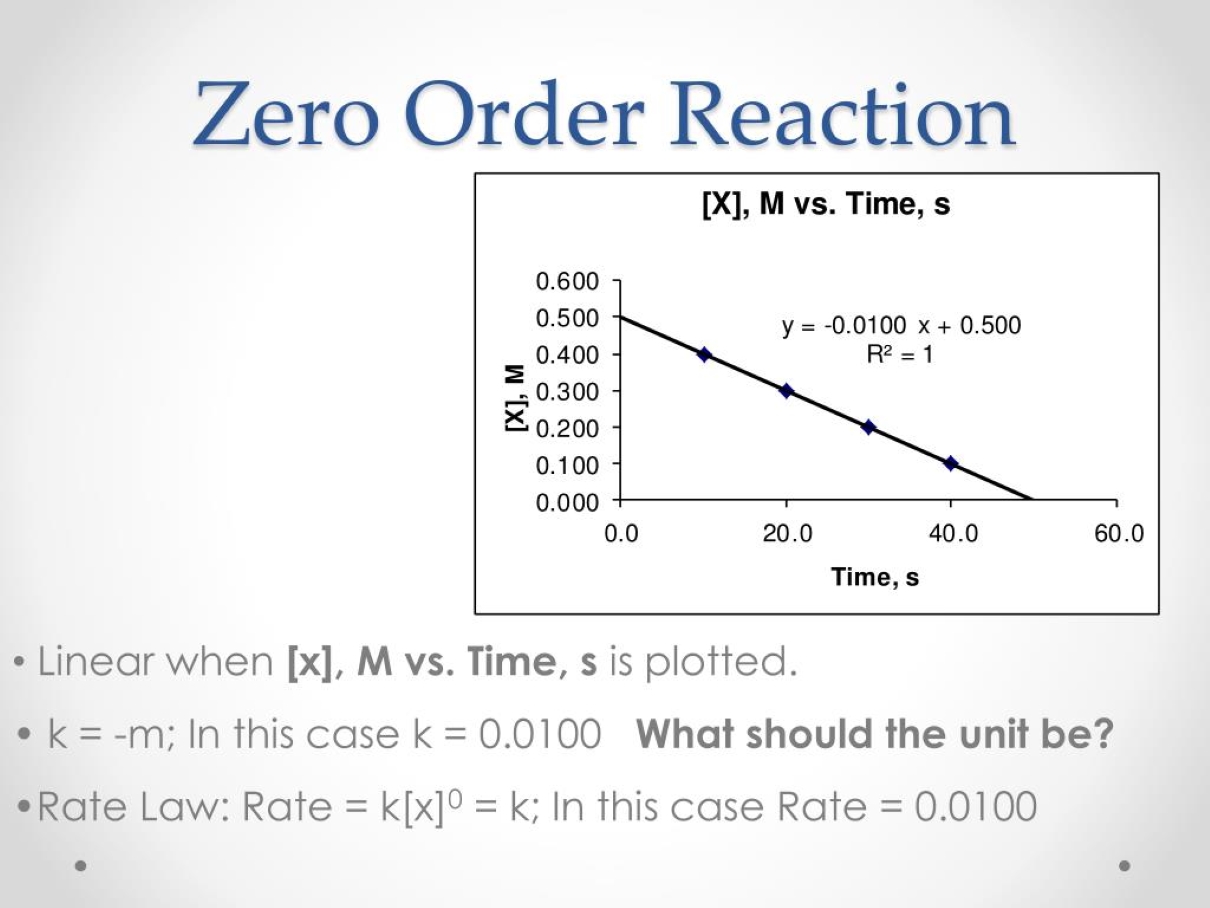
Zero Order Reaction Kinetics . On a tungsten surface, however, the. For this reason, reactions that follow. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. Chemical reactions may be assigned reaction orders that describe their kinetics. We refer to these reactions as zeroth order because we could also.
from facts.net
On a tungsten surface, however, the. We refer to these reactions as zeroth order because we could also. For this reason, reactions that follow. Chemical reactions may be assigned reaction orders that describe their kinetics. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. Its differential rate law is rate = k.
19 Mindblowing Facts About ZeroOrder Reaction
Zero Order Reaction Kinetics Its differential rate law is rate = k. Its differential rate law is rate = k. We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. On a tungsten surface, however, the. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power.
From www.youtube.com
zero order reaction and examples (chemical part 20 for CBSE Zero Order Reaction Kinetics If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. On a tungsten surface, however, the. Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. We refer to these. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Zero Order Reaction Kinetics On a tungsten surface, however, the. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. We refer to these reactions as zeroth order because we could also. Its differential rate law is rate = k. Chemical. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Zero Order Reaction Kinetics On a tungsten surface, however, the. Chemical reactions may be assigned reaction orders that describe their kinetics. We refer to these reactions as zeroth order because we could also. Its differential rate law is rate = k. For this reason, reactions that follow. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From pediaa.com
Difference Between First Order and Zero Order Definition Zero Order Reaction Kinetics Chemical reactions may be assigned reaction orders that describe their kinetics. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. We refer to these reactions as zeroth order because we could also. For this reason, reactions. Zero Order Reaction Kinetics.
From www.researchgate.net
Example of zero order Download Scientific Diagram Zero Order Reaction Kinetics If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. On a tungsten surface, however, the. We refer to these. Zero Order Reaction Kinetics.
From www.youtube.com
Zero Order Reaction Chemical Chemistry Class 12 YouTube Zero Order Reaction Kinetics On a tungsten surface, however, the. For this reason, reactions that follow. Chemical reactions may be assigned reaction orders that describe their kinetics. We refer to these reactions as zeroth order because we could also. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From www.youtube.com
Derivation of Zero order Integrated rate equation(chemical Zero Order Reaction Kinetics If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. Chemical reactions may be assigned reaction orders that describe their kinetics. On a tungsten surface, however, the. We refer to these reactions as zeroth order because we. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Zero Order Reaction Kinetics Its differential rate law is rate = k. We refer to these reactions as zeroth order because we could also. For this reason, reactions that follow. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. On. Zero Order Reaction Kinetics.
From www.researchgate.net
Zeroorder reaction for production of RNS (HNO 3(aq) and HNO Zero Order Reaction Kinetics On a tungsten surface, however, the. Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. We refer to these reactions as zeroth order because we could also. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From www.youtube.com
15) Zero order reaction Integrated rate law Examples of Zero order Zero Order Reaction Kinetics We refer to these reactions as zeroth order because we could also. On a tungsten surface, however, the. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. For this reason, reactions that follow. Its differential rate. Zero Order Reaction Kinetics.
From www.youtube.com
Derivation of Half life period of Zero order reaction(chemical Zero Order Reaction Kinetics For this reason, reactions that follow. We refer to these reactions as zeroth order because we could also. On a tungsten surface, however, the. Chemical reactions may be assigned reaction orders that describe their kinetics. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times. Zero Order Reaction Kinetics.
From www.youtube.com
CHEMICAL integrated rate equation for zero order reaction Zero Order Reaction Kinetics Its differential rate law is rate = k. For this reason, reactions that follow. We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. On a tungsten surface, however, the. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From www.youtube.com
order reaction (derivation of units for the order of Zero Order Reaction Kinetics If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. We refer to these reactions as zeroth order because we. Zero Order Reaction Kinetics.
From www.youtube.com
zero order reaction and its examples(chemical part 16 for CBSE Zero Order Reaction Kinetics For this reason, reactions that follow. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. Chemical reactions may be assigned reaction orders that describe their kinetics. We refer. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Zero Order Reaction Kinetics Its differential rate law is rate = k. For this reason, reactions that follow. We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. On a tungsten surface, however, the. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Zero Order Reaction Kinetics For this reason, reactions that follow. Its differential rate law is rate = k. Chemical reactions may be assigned reaction orders that describe their kinetics. On a tungsten surface, however, the. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT Summary of the of ZeroOrder, FirstOrder and Second Zero Order Reaction Kinetics Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. On a tungsten surface, however, the. We refer to these reactions as zeroth order because we could also. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From www.youtube.com
half life of zero order reaction(chemical part 48 for CBSE Zero Order Reaction Kinetics For this reason, reactions that follow. Chemical reactions may be assigned reaction orders that describe their kinetics. We refer to these reactions as zeroth order because we could also. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT ZeroOrder Release From Capsule Reservoirs through Semi Zero Order Reaction Kinetics On a tungsten surface, however, the. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. We refer to these reactions as zeroth order because we could also. Chemical. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT 144 ZeroOrder Reactions PowerPoint Presentation, free download Zero Order Reaction Kinetics For this reason, reactions that follow. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. Its differential rate law is rate = k. We refer to these reactions as zeroth order because we could also. On. Zero Order Reaction Kinetics.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Zero Order Reaction Kinetics We refer to these reactions as zeroth order because we could also. On a tungsten surface, however, the. For this reason, reactions that follow. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. Chemical reactions may. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2054453 Zero Order Reaction Kinetics On a tungsten surface, however, the. We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant. Zero Order Reaction Kinetics.
From www.youtube.com
CHEMISTRY 201 from graphs for a zero order reaction YouTube Zero Order Reaction Kinetics If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. We refer to these reactions as zeroth order because we could also. For this reason, reactions that follow. On a tungsten surface, however, the. Chemical reactions may. Zero Order Reaction Kinetics.
From facts.net
19 Mindblowing Facts About ZeroOrder Reaction Zero Order Reaction Kinetics For this reason, reactions that follow. On a tungsten surface, however, the. Chemical reactions may be assigned reaction orders that describe their kinetics. Its differential rate law is rate = k. We refer to these reactions as zeroth order because we could also. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From general.chemistrysteps.com
ZeroOrder Reactions Chemistry Steps Zero Order Reaction Kinetics Chemical reactions may be assigned reaction orders that describe their kinetics. Its differential rate law is rate = k. We refer to these reactions as zeroth order because we could also. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a. Zero Order Reaction Kinetics.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical Zero Order Reaction Kinetics We refer to these reactions as zeroth order because we could also. Its differential rate law is rate = k. Chemical reactions may be assigned reaction orders that describe their kinetics. On a tungsten surface, however, the. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant. Zero Order Reaction Kinetics.
From www.youtube.com
Zero Order Reaction Chemical 04 Class 12/JEE/NEET Zero Order Reaction Kinetics On a tungsten surface, however, the. We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times. Zero Order Reaction Kinetics.
From www.youtube.com
graph of integrated rate equation of zero order reaction (chemical Zero Order Reaction Kinetics We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. On a tungsten surface, however, the. For this reason, reactions that follow. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times. Zero Order Reaction Kinetics.
From www.youtube.com
Zero Order Reaction Integrated Rate constant equation for zero order Zero Order Reaction Kinetics Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. We refer to these reactions as zeroth order because we could also. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the. Zero Order Reaction Kinetics.
From www.youtube.com
Worked example Zero Order Reaction Chemistry Khan Zero Order Reaction Kinetics On a tungsten surface, however, the. Its differential rate law is rate = k. We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. For this reason, reactions that follow. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From www.youtube.com
Zeroorder reactions AP Chemistry Khan Academy YouTube Zero Order Reaction Kinetics If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. Its differential rate law. Zero Order Reaction Kinetics.
From scienceinfo.com
Zeroorder Reaction Rate Equation, Unit, Graph, Example Zero Order Reaction Kinetics Chemical reactions may be assigned reaction orders that describe their kinetics. On a tungsten surface, however, the. Its differential rate law is rate = k. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. We refer. Zero Order Reaction Kinetics.
From www.youtube.com
What is a zero order reaction ? Chemistry Khan Academy Zero Order Reaction Kinetics On a tungsten surface, however, the. For this reason, reactions that follow. Its differential rate law is rate = k. We refer to these reactions as zeroth order because we could also. Chemical reactions may be assigned reaction orders that describe their kinetics. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Zero Order Reaction Kinetics Chemical reactions may be assigned reaction orders that describe their kinetics. On a tungsten surface, however, the. For this reason, reactions that follow. Its differential rate law is rate = k. We refer to these reactions as zeroth order because we could also. If it's zero order with respect to a, we can write that the rate of the reaction. Zero Order Reaction Kinetics.
From chemistnotes.com
Zero order reaction Definition, integrated rate law, characteristics Zero Order Reaction Kinetics Its differential rate law is rate = k. Chemical reactions may be assigned reaction orders that describe their kinetics. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the rate constant k, times the concentration of a to the zero power. On a tungsten surface, however, the. For this. Zero Order Reaction Kinetics.