Medical Device Lifecycle Management . The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Manage quality throughout the life cycle of a. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring.
from toolbox.eupati.eu
The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. Manage quality throughout the life cycle of a. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring.
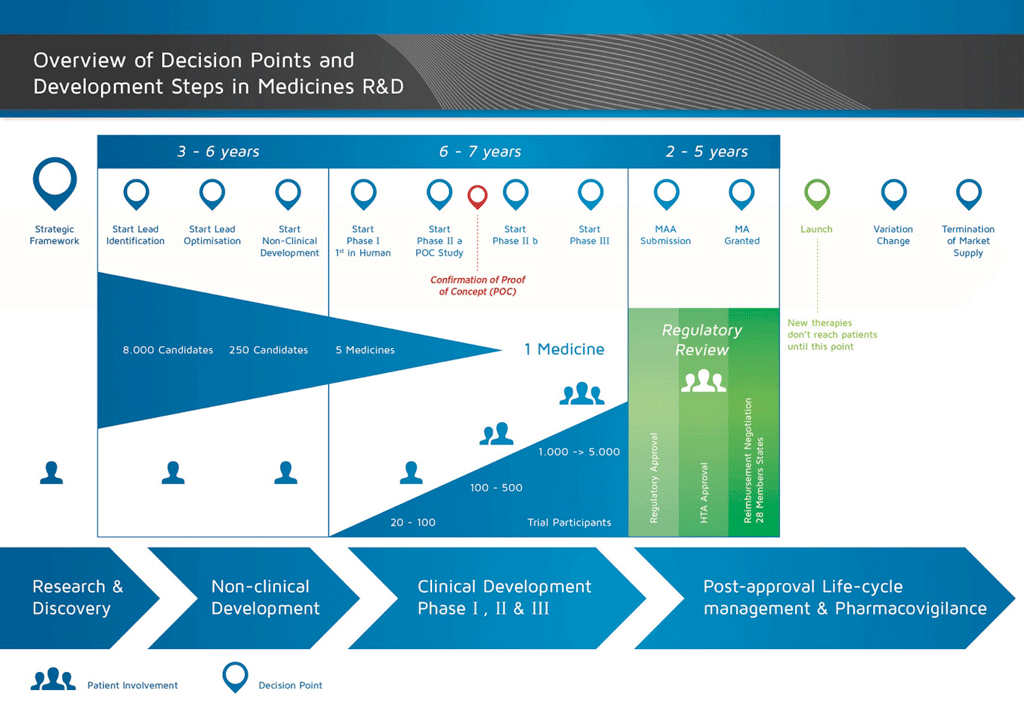
Making a medicine. Step 10 Lifecycle management EUPATI Toolbox
Medical Device Lifecycle Management A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. Manage quality throughout the life cycle of a. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,.
From toolbox.eupati.eu
Making a medicine. Step 10 Lifecycle management EUPATI Toolbox Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. Manage quality throughout the life cycle of a. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. The. Medical Device Lifecycle Management.
From www.vrogue.co
Understanding The Medical Device Product Lifecycle Dy vrogue.co Medical Device Lifecycle Management The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Learn how to embark on a digital transformation journey uniquely designed. Medical Device Lifecycle Management.
From www.tuleap.org
Key features of a top Application Lifecycle Management (ALM) tool • Tuleap Medical Device Lifecycle Management Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. Manage quality throughout the life cycle of a. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Learn how to. Medical Device Lifecycle Management.
From www.researchgate.net
(PDF) A New Approach of System Engineering to Medical Device Lifecycle Management Medical Device Lifecycle Management A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle. Medical Device Lifecycle Management.
From enterprisepeak.com
Medical Devices from Concept to Commercialization Enterprise Peak Medical Device Lifecycle Management The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Navigating. Medical Device Lifecycle Management.
From h-isac.org
Medical Device Cybersecurity Lifecycle Management Medical Device Lifecycle Management Manage quality throughout the life cycle of a. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. The total product life cycle (tplc) approach promotes and enhances. Medical Device Lifecycle Management.
From www.greenlight.guru
Medical Device Development [Understanding the 5 Phases] Medical Device Lifecycle Management The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The total product life cycle (tplc) approach promotes. Medical Device Lifecycle Management.
From www.mobilecorp.com.au
Device Lifecycle Management. From Procurement To EOL. Medical Device Lifecycle Management A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle. Medical Device Lifecycle Management.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 Medical Device Lifecycle Management The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. A free brochure with tips for getting started with iso 13485,. Medical Device Lifecycle Management.
From 24x7mag.com
The Medical Device Security Life Cycle 24x7 Medical Device Lifecycle Management Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Manage quality throughout the life cycle of a. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Learn how to. Medical Device Lifecycle Management.
From www.deskera.com
Ultimate Guide to Product Lifecycle Management Medical Device Lifecycle Management A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. The model allows the manufacturer to. Medical Device Lifecycle Management.
From www.vrogue.co
How To Manage The Diagnostic Medical Device Technolog vrogue.co Medical Device Lifecycle Management Manage quality throughout the life cycle of a. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. A free brochure. Medical Device Lifecycle Management.
From 43.204.27.49
A Complete Guide to Effective Device Lifecycle Management The Hybr1d Blog Medical Device Lifecycle Management The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Manage quality throughout the life cycle of a. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The model allows the manufacturer to support business management and the complete time frame of. Medical Device Lifecycle Management.
From www.qmswrapper.com
What is medical devices lifecycle qmsWrapper Medical Device Lifecycle Management Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. Manage quality throughout the life cycle of a. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle. Medical Device Lifecycle Management.
From qbdgroup.com
From idea to post market surveillance the phases of the medical device lifecycle QbD Group Medical Device Lifecycle Management Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The model allows the manufacturer to support business management and the. Medical Device Lifecycle Management.
From www.wbm.ca
Device Lifecycle Management Service WBM Technologies Medical Device Lifecycle Management The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Manage quality throughout the life cycle of a. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. A free brochure. Medical Device Lifecycle Management.
From aganamed.com
Educational Literature AganaMed LLC Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Manage. Medical Device Lifecycle Management.
From htecgroup.com
Risk Management in the Medical Device Lifecycle Development and Testing HTEC Medical Device Lifecycle Management Manage quality throughout the life cycle of a. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The model allows the manufacturer to support business management and. Medical Device Lifecycle Management.
From pnghut.com
Product Lifecycle Management Medical Device Lifecycle Manufacturing Brand Development Cycle Medical Device Lifecycle Management Manage quality throughout the life cycle of a. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. The total product life cycle (tplc) approach promotes and enhances. Medical Device Lifecycle Management.
From www.kmcsystems.com
KMC Medical Device Development Medical Product Lifecycle Management Medical Device Lifecycle Management Manage quality throughout the life cycle of a. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. The model allows the manufacturer to support business management and. Medical Device Lifecycle Management.
From www.researchgate.net
Medical device lifecycle management (see online version for colours) Download Scientific Diagram Medical Device Lifecycle Management Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. A free brochure with tips for getting started with iso 13485,. Medical Device Lifecycle Management.
From touchcom.ie
Device Life Cycle Management Mobile Device Management Medical Device Lifecycle Management A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Manage quality throughout the life cycle of a. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle. Medical Device Lifecycle Management.
From www.imei.com.au
Devicelifecyclemanagement Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The model allows the manufacturer to support business management. Medical Device Lifecycle Management.
From mavink.com
Medical Device Life Cycle Phases Medical Device Lifecycle Management The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Manage quality throughout the life cycle of a. Learn how to embark on a digital transformation journey uniquely designed for the medical device. Medical Device Lifecycle Management.
From www.vrogue.co
The Life Cycle Of Medical Care Processes Download Sci vrogue.co Medical Device Lifecycle Management The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. Manage quality throughout the life cycle of a. The total product life cycle (tplc) approach promotes and enhances. Medical Device Lifecycle Management.
From www.who.int
Trainings Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. Manage quality throughout the life cycle of a. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. The. Medical Device Lifecycle Management.
From qbdgroup.com
MEDICAL DEVICE GUIDE The Pathway from Idea to Patient QbD Group Medical Device Lifecycle Management A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Manage quality throughout the life cycle of a. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,.. Medical Device Lifecycle Management.
From www.researchgate.net
The LifeCycle of a Medical Device. Download Scientific Diagram Medical Device Lifecycle Management Manage quality throughout the life cycle of a. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. A free brochure. Medical Device Lifecycle Management.
From mavink.com
Mobile Device Life Cycle Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. The total product life cycle (tplc) approach promotes and enhances the transparency, efficiency, and agility of the. Manage quality throughout the life cycle of a. A. Medical Device Lifecycle Management.
From www.koveninnovation.com
Ultimate Guide to Risk Management in Medical Devices Koven cardiovascular innovation Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to. Medical Device Lifecycle Management.
From www.researchgate.net
Medical device lifecycle management (see online version for colours) Download Scientific Diagram Medical Device Lifecycle Management A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. Learn how to embark on a digital transformation. Medical Device Lifecycle Management.
From www.joharidigital.com
Understanding The 7 Phases of Medical Device Development & Manufacturing Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Manage quality throughout the life cycle of a. Navigating the medical device life cycle requires meticulous planning, execution,. Medical Device Lifecycle Management.
From www.researchgate.net
Medical device lifecycle management (see online version for colours) Download Scientific Diagram Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. The model allows the manufacturer to support business management and the complete time frame of all activities associated. Medical Device Lifecycle Management.
From www.itjones.com
Device Lifecycle Management And The Future Of Global Employee Mobility Jones IT Medical Device Lifecycle Management The model allows the manufacturer to support business management and the complete time frame of all activities associated with design,. Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. Manage quality throughout the life cycle of a. The total product life cycle (tplc) approach promotes and enhances. Medical Device Lifecycle Management.
From www.slideshare.net
Simplifying Medical Device Lifecycle Management PPT Medical Device Lifecycle Management Learn how to embark on a digital transformation journey uniquely designed for the medical device industry with ibm engineering lifecycle management solutions. Navigating the medical device life cycle requires meticulous planning, execution, and monitoring. A free brochure with tips for getting started with iso 13485, requirements for quality management systems related to medical devices. Manage quality throughout the life cycle. Medical Device Lifecycle Management.