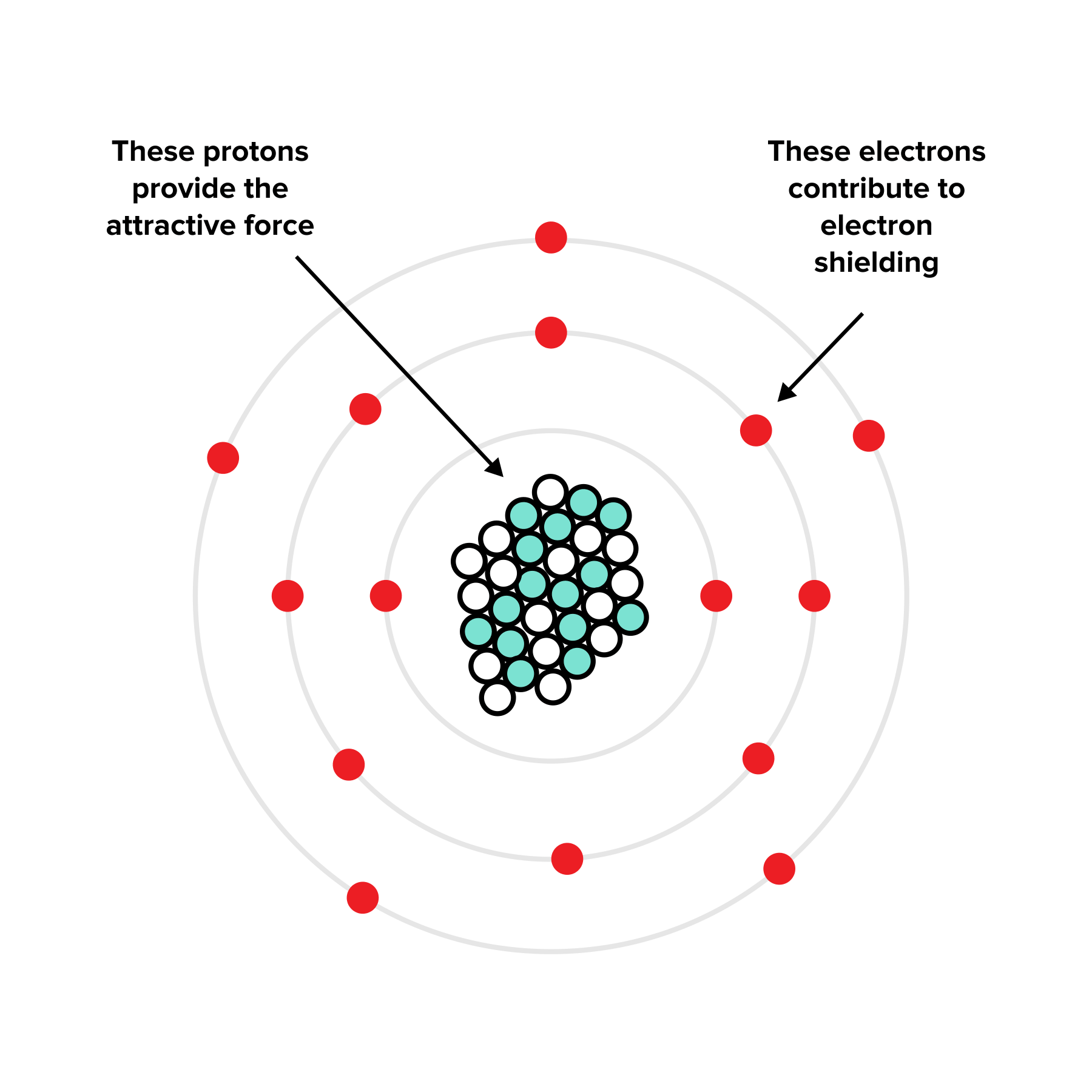
Electron Shielding And Effective Nuclear Charge . The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. For example, let’s compare the effective nuclear charges of li and fluorine: Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. This reduced charge is known as the effective nuclear charge. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. It can be approximated by the equation: The effective nuclear charge is the net positive charge experienced by valence electrons.
from www.shemmassianconsulting.com
Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. This reduced charge is known as the effective nuclear charge. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge is the net positive charge experienced by valence electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. It can be approximated by the equation: For example, let’s compare the effective nuclear charges of li and fluorine:
Atoms and Periodic Trends for the MCAT Everything You Need to Know
Electron Shielding And Effective Nuclear Charge This reduced charge is known as the effective nuclear charge. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. It can be approximated by the equation: For example, let’s compare the effective nuclear charges of li and fluorine: This reduced charge is known as the effective nuclear charge. Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. The effective nuclear charge is the net positive charge experienced by valence electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,.
From chem.libretexts.org
1.15 Effective Nuclear Charge and Shielding Chemistry LibreTexts Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. It can be approximated by the equation: For example, let’s compare the effective nuclear charges of li and fluorine:. Electron Shielding And Effective Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps Electron Shielding And Effective Nuclear Charge Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. It can be approximated by the equation: In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced. Electron Shielding And Effective Nuclear Charge.
From www.studypool.com
SOLUTION Electron configurations of cations and anions shielding Electron Shielding And Effective Nuclear Charge The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. The effective nuclear charge is the net positive charge experienced by valence electrons. It can be approximated by the equation: For example, let’s compare the effective nuclear charges of li and fluorine:. Electron Shielding And Effective Nuclear Charge.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE Electron Shielding And Effective Nuclear Charge It can be approximated by the equation: The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. The effective nuclear charge is the net positive charge experienced by valence electrons. For example, let’s compare the effective nuclear charges of li and fluorine:. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Quantum Theory PowerPoint Presentation, free download ID1185278 Electron Shielding And Effective Nuclear Charge Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to. Electron Shielding And Effective Nuclear Charge.
From favpng.com
Effective Nuclear Charge Shielding Effect Atomic Nucleus Atomic Number Electron Shielding And Effective Nuclear Charge In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge may be defined as the actual nuclear charge. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 6 Periodic Trends PowerPoint Presentation ID6147255 Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. For example, let’s compare the effective nuclear charges of li and fluorine: It can be approximated by the equation: This reduced charge is known as the effective nuclear charge. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused. Electron Shielding And Effective Nuclear Charge.
From www.numerade.com
SOLVEDDefine shielding and effective nuclear charge. What is the Electron Shielding And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. It can be approximated by the equation: The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. The effective nuclear charge is. Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube Electron Shielding And Effective Nuclear Charge Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. For example, let’s compare the effective nuclear charges of li and fluorine: This reduced charge is known as the effective nuclear charge. The effective nuclear charge is the net positive charge experienced by. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT ATOMIC ELECTRON CONFIGURATIONS AND ORBITAL SHAPES PowerPoint Electron Shielding And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. For example, let’s compare the effective nuclear charges of li and fluorine: The effective nuclear charge is the net positive charge experienced by valence electrons. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra Electron Shielding And Effective Nuclear Charge The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. It can be approximated by the equation: In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. For example, let’s compare the effective nuclear charges of li and fluorine: This reduced charge is known as the effective nuclear charge. Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective. Electron Shielding And Effective Nuclear Charge.
From slideplayer.com
Development of the Periodic Table ppt download Electron Shielding And Effective Nuclear Charge It can be approximated by the equation: For example, let’s compare the effective nuclear charges of li and fluorine: The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. This reduced charge is known as the effective nuclear charge. The concept of. Electron Shielding And Effective Nuclear Charge.
From socratic.org
How are shielding effect and atomic radius related? Socratic Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. For example, let’s compare the effective nuclear charges of li and fluorine: The effective nuclear charge may be defined. Electron Shielding And Effective Nuclear Charge.
From www.numerade.com
SOLVEDSlater's rules are a simple way to estimate the effective Electron Shielding And Effective Nuclear Charge It can be approximated by the equation: In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. The effective nuclear charge is the net positive charge experienced by valence electrons. For example, let’s compare the effective nuclear charges of li and fluorine:. Electron Shielding And Effective Nuclear Charge.
From slideplayer.com
Unit 3 Chapter ppt download Electron Shielding And Effective Nuclear Charge It can be approximated by the equation: Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge is the net positive charge experienced by valence electrons.. Electron Shielding And Effective Nuclear Charge.
From www.breakingatom.com
Nuclear Charge Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. This reduced charge is known as the effective nuclear charge. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect. Electron Shielding And Effective Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. For example, let’s compare the effective nuclear charges of li and fluorine: The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to. Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
How To Calculate The Effective Nuclear Charge of an Electron YouTube Electron Shielding And Effective Nuclear Charge Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. The effective nuclear charge is. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Electron Shielding And Effective Nuclear Charge Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge is the net positive charge experienced by valence electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Yes, the number of valence electrons increases. Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
Electron Shielding and Effective Nuclear Charge (Zeff) YouTube Electron Shielding And Effective Nuclear Charge In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. The effective nuclear charge may. Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube Electron Shielding And Effective Nuclear Charge For example, let’s compare the effective nuclear charges of li and fluorine: In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,.. Electron Shielding And Effective Nuclear Charge.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Shielding And Effective Nuclear Charge In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. Yes, the number of valence electrons increases as well, but these have. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Atomic Radii PowerPoint Presentation, free download ID6448698 Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. It can be approximated by the equation: This reduced charge is known as the effective nuclear charge. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. Overall, the. Electron Shielding And Effective Nuclear Charge.
From www.shemmassianconsulting.com
Atoms and Periodic Trends for the MCAT Everything You Need to Know Electron Shielding And Effective Nuclear Charge Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. This reduced charge is known as the effective nuclear charge. The effective. Electron Shielding And Effective Nuclear Charge.
From byjus.com
Explain effective nuclear charge shielding effect Factor on which Electron Shielding And Effective Nuclear Charge Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. The concept of electron shielding, in which intervening electrons act to reduce. Electron Shielding And Effective Nuclear Charge.
From www.breakingatom.com
Nuclear charge Definition Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. This reduced charge is known as the effective nuclear charge. In this section, we explore one model for quantitatively. Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube Electron Shielding And Effective Nuclear Charge Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. This reduced charge is known as the effective nuclear charge. Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. The effective nuclear. Electron Shielding And Effective Nuclear Charge.
From www.studypool.com
SOLUTION Electron configurations of cations and anions shielding Electron Shielding And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. This reduced charge is known as the effective nuclear charge. Yes, the. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Unit 8 Periodic Properties of the Elements PowerPoint Electron Shielding And Effective Nuclear Charge It can be approximated by the equation: The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Effective Nuclear Charge PowerPoint Presentation, free download Electron Shielding And Effective Nuclear Charge Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. For example, let’s compare the effective nuclear charges of li and fluorine: The. Electron Shielding And Effective Nuclear Charge.
From gamma.app
Understanding Shielding Effect and Effective Nuclear Charge Electron Shielding And Effective Nuclear Charge The effective nuclear charge is the net positive charge experienced by valence electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. For example, let’s compare the effective nuclear charges of li and fluorine: Yes, the number of valence electrons increases as well, but these have a significantly lower. Electron Shielding And Effective Nuclear Charge.
From slideplayer.com
Effective Nuclear Charge, Shielding, and Trends on the Periodic Table Electron Shielding And Effective Nuclear Charge Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge. This reduced charge is known as the effective nuclear charge. Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. For example, let’s. Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Why “periodic?” PowerPoint Presentation, free download ID1779615 Electron Shielding And Effective Nuclear Charge In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. The effective nuclear charge may be defined as the actual nuclear charge (z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron. Overall, the outer electrons. Electron Shielding And Effective Nuclear Charge.
From www.learner.org
Organizing Atoms and Electrons The Periodic Table Annenberg Learner Electron Shielding And Effective Nuclear Charge Overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an. This reduced charge is known as the effective nuclear charge. The concept. Electron Shielding And Effective Nuclear Charge.