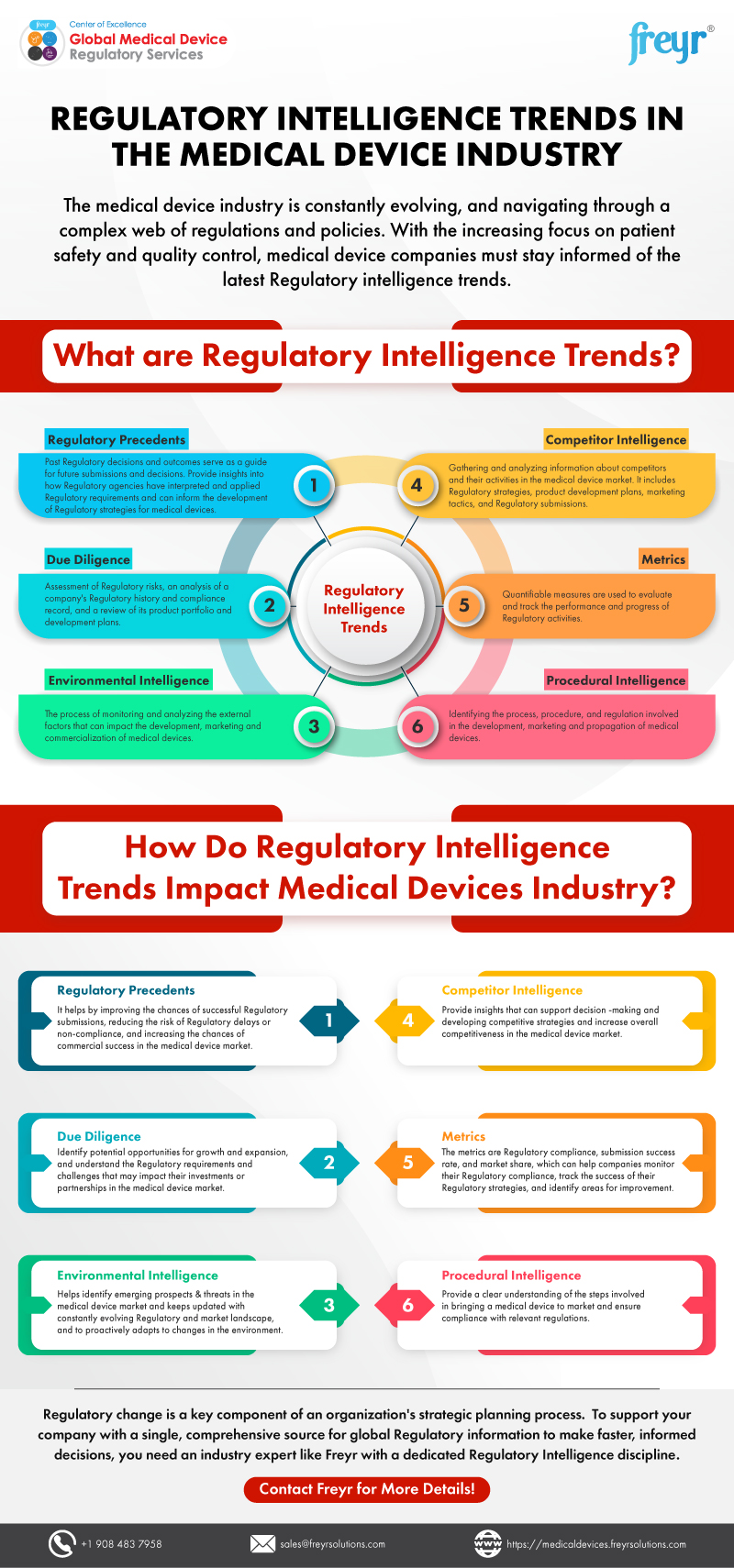
Medical Device Regulatory Intelligence Tools . Find the relevant information you need to respond instead of react to change all while remaining compliant. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. Access and compare requirements for over 110 countries, regions, and. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; To better organize this data, we will break it down into five. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage.
from www.freyrsolutions.com
Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Access and compare requirements for over 110 countries, regions, and. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. To better organize this data, we will break it down into five. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. Find the relevant information you need to respond instead of react to change all while remaining compliant. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory.
Regulatory Intelligence Trends in the Medical Device Industry Freyr
Medical Device Regulatory Intelligence Tools To better organize this data, we will break it down into five. Access and compare requirements for over 110 countries, regions, and. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. Find the relevant information you need to respond instead of react to change all while remaining compliant. To better organize this data, we will break it down into five. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. Access to regulatory requirements for human drugs, biologics, medical devices and ivds;
From cashier.mijndomein.nl
Medical Device Regulatory Strategy Template Medical Device Regulatory Intelligence Tools Find the relevant information you need to respond instead of react to change all while remaining compliant. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. To better organize this data, we will break it down into five. Access to regulatory requirements for human drugs, biologics, medical devices and ivds;. Medical Device Regulatory Intelligence Tools.
From www.qualitiso.com
Doing a Regulatory Watch for medical devices Medical Device Regulatory Intelligence Tools It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. Access and compare requirements for over. Medical Device Regulatory Intelligence Tools.
From www.frontiersin.org
Frontiers Advancing Regulatory Science With Computational Modeling Medical Device Regulatory Intelligence Tools By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. This article discusses how medical device guidance documents, regulations,. Medical Device Regulatory Intelligence Tools.
From www.freyrsolutions.com
Understanding Regulatory Intelligence (RI) in Medical Devices Freyr Medical Device Regulatory Intelligence Tools Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Find the relevant information you need to respond instead of react to change all while remaining compliant. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. This article discusses how medical device guidance documents,. Medical Device Regulatory Intelligence Tools.
From easymedicaldevice.com
Best Quality and Regulatory Affairs Tools (Medical Devices) Medical Device Regulatory Intelligence Tools Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. Find the relevant information you need to respond instead of react to change all while remaining compliant. Access to regulatory requirements. Medical Device Regulatory Intelligence Tools.
From www.quantib.com
A 101 guide to the FDA regulatory process for AI radiology software Medical Device Regulatory Intelligence Tools It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. This article discusses how medical device guidance documents, regulations,. Medical Device Regulatory Intelligence Tools.
From www.hardianhealth.com
Medical Device Regulatory Services UKCA, CE and FDA — Hardian Health Medical Device Regulatory Intelligence Tools Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. To better organize this data, we will break it down into five. Find the relevant information you need to respond instead of react to change all while remaining compliant. Access and compare requirements for over 110 countries, regions, and. Access to. Medical Device Regulatory Intelligence Tools.
From www.regdesk.co
Medical Device Regulatory Intelligence RegDesk Medical Device Regulatory Intelligence Tools Find the relevant information you need to respond instead of react to change all while remaining compliant. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in. Medical Device Regulatory Intelligence Tools.
From www.rimsys.io
Medical Device Regulatory Intelligence Rimsys MedTech RIM Software Medical Device Regulatory Intelligence Tools By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. This article discusses how medical device guidance documents, regulations, standards and requirements are. Medical Device Regulatory Intelligence Tools.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Medical Device Regulatory Intelligence Tools This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. It is vital that these tools remain agile and fit for use. Medical Device Regulatory Intelligence Tools.
From qbdgroup.com
Regulatory Intelligence for Medical Devices Medical Device Regulatory Intelligence Tools By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. Find the relevant information you need to respond instead of react to change all while remaining compliant. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in. Medical Device Regulatory Intelligence Tools.
From blog.sierralabs.com
8 Regulatory Strategy Guidelines for Your Medical Device Medical Device Regulatory Intelligence Tools This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. It is vital that these tools remain agile and fit for use. Medical Device Regulatory Intelligence Tools.
From template.mapadapalavra.ba.gov.br
Medical Device Regulatory Strategy Template Medical Device Regulatory Intelligence Tools Find the relevant information you need to respond instead of react to change all while remaining compliant. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. By providing access to. Medical Device Regulatory Intelligence Tools.
From blog.sierralabs.com
6 Regulatory Pathways to Bring Your Medical Device to Market Medical Device Regulatory Intelligence Tools Find the relevant information you need to respond instead of react to change all while remaining compliant. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. It is vital that. Medical Device Regulatory Intelligence Tools.
From secure.constellation.iqvia.com
Regulatory Intelligence Medical Device Regulatory Intelligence Tools This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. To better organize this data, we will break it down into five. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. By providing access to regulatory. Medical Device Regulatory Intelligence Tools.
From tentoplus.com
Medical Device Regulatory Compliance Software Medical Device Regulatory Intelligence Tools Find the relevant information you need to respond instead of react to change all while remaining compliant. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Access and compare requirements for over 110 countries, regions, and. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence. Medical Device Regulatory Intelligence Tools.
From template.mapadapalavra.ba.gov.br
Medical Device Regulatory Strategy Template Medical Device Regulatory Intelligence Tools To better organize this data, we will break it down into five. Find the relevant information you need to respond instead of react to change all while remaining compliant. Access and compare requirements for over 110 countries, regions, and. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence. Medical Device Regulatory Intelligence Tools.
From www.regdesk.co
RegDesk Regulatory Intelligence Software RIMS RegDesk Medical Device Regulatory Intelligence Tools Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. To better organize this data, we will break it. Medical Device Regulatory Intelligence Tools.
From gh.bmj.com
Regulatory reliance for convergence and harmonisation in the medical Medical Device Regulatory Intelligence Tools Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Access and compare requirements for over 110 countries, regions, and. Find the relevant information you need to respond instead of react to change all while remaining compliant. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. This article. Medical Device Regulatory Intelligence Tools.
From lfhregulatory.co.uk
Medical Device Regulatory Intelligence Medical Device Regulatory Intelligence Tools Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Access and compare requirements for over 110 countries, regions, and. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. This article discusses how medical device guidance documents, regulations, standards and requirements are presented in. Medical Device Regulatory Intelligence Tools.
From data1.skinnyms.com
Regulatory Strategy Template For Medical Devices Medical Device Regulatory Intelligence Tools Find the relevant information you need to respond instead of react to change all while remaining compliant. To better organize this data, we will break it down into five. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. By providing access to regulatory information and analysis,. Medical Device Regulatory Intelligence Tools.
From www.sycaimedical.com
Overview on the regulatory path for software medical devices Medical Device Regulatory Intelligence Tools This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage.. Medical Device Regulatory Intelligence Tools.
From starfishmedical.com
Regulatory Science Tools Reduce Risk in New Medical Devices Medical Device Regulatory Intelligence Tools Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Find the relevant information you need to respond instead of react to change all while remaining compliant. Access and compare requirements for over 110 countries, regions, and. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and. Medical Device Regulatory Intelligence Tools.
From www.youtube.com
Regulatory Standards & Risk Management in Medical Devices YouTube Medical Device Regulatory Intelligence Tools By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. Access and compare requirements for over 110 countries, regions, and. To better organize this data, we will break it down into five. Access to regulatory requirements for human drugs, biologics, medical devices. Medical Device Regulatory Intelligence Tools.
From www.mdpi.com
Biomedicines Free FullText Expanding Quality by Design Principles Medical Device Regulatory Intelligence Tools By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Access and compare requirements for over 110 countries, regions, and. It is vital that these tools remain agile and fit. Medical Device Regulatory Intelligence Tools.
From www.heartlungcirc.org
Regulatory Requirements For Medical Devices And Vascular Ageing An Medical Device Regulatory Intelligence Tools By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. Find the relevant information you need to respond instead. Medical Device Regulatory Intelligence Tools.
From www.medicaldevice.expert
Regulatory Strategy Medical Device Expert News Medical Device Regulatory Intelligence Tools Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. It. Medical Device Regulatory Intelligence Tools.
From www.rimsys.io
Medical Device Regulatory Intelligence Rimsys MedTech RIM Software Medical Device Regulatory Intelligence Tools Find the relevant information you need to respond instead of react to change all while remaining compliant. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. To better organize this data, we will break. Medical Device Regulatory Intelligence Tools.
From www.rimsys.io
Medical Device Regulatory Intelligence Rimsys MedTech RIM Software Medical Device Regulatory Intelligence Tools To better organize this data, we will break it down into five. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; It is vital that these tools remain agile and fit for use in each of the functions. Medical Device Regulatory Intelligence Tools.
From www.freyrsolutions.com
Regulatory Intelligence Trends in the Medical Device Industry Freyr Medical Device Regulatory Intelligence Tools It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. To better organize this data,. Medical Device Regulatory Intelligence Tools.
From www.freyrsolutions.com
Compliance Risk Rating Scale for Medical Devices Freyr Global Medical Device Regulatory Intelligence Tools Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage. This. Medical Device Regulatory Intelligence Tools.
From operonstrategist.com
How to Choose a Medical Device Regulatory Consultants? (Guidance for Medical Device Regulatory Intelligence Tools Find the relevant information you need to respond instead of react to change all while remaining compliant. Access and compare requirements for over 110 countries, regions, and. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. To better organize this data, we will break it down into five. Access to. Medical Device Regulatory Intelligence Tools.
From www.presentationeze.com
European Medical Device Regulatory Approval Process PresentationEZE Medical Device Regulatory Intelligence Tools Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. It is vital that these tools remain agile and fit for use in each of the functions of the regulatory intelligence life cycle. By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development,. Medical Device Regulatory Intelligence Tools.
From qbdgroup.com
Artificial Intelligence in Medical Devices what do we know so far? QbD Medical Device Regulatory Intelligence Tools To better organize this data, we will break it down into five. Regdesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; Access and compare requirements for over 110 countries, regions, and. This article discusses how medical device guidance. Medical Device Regulatory Intelligence Tools.
From www.frohberg.de
Managing Medical Devices within a Regulatory Framework EBook Medical Device Regulatory Intelligence Tools This article discusses how medical device guidance documents, regulations, standards and requirements are presented in increasing amounts and how regulatory. Access to regulatory requirements for human drugs, biologics, medical devices and ivds; By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage.. Medical Device Regulatory Intelligence Tools.