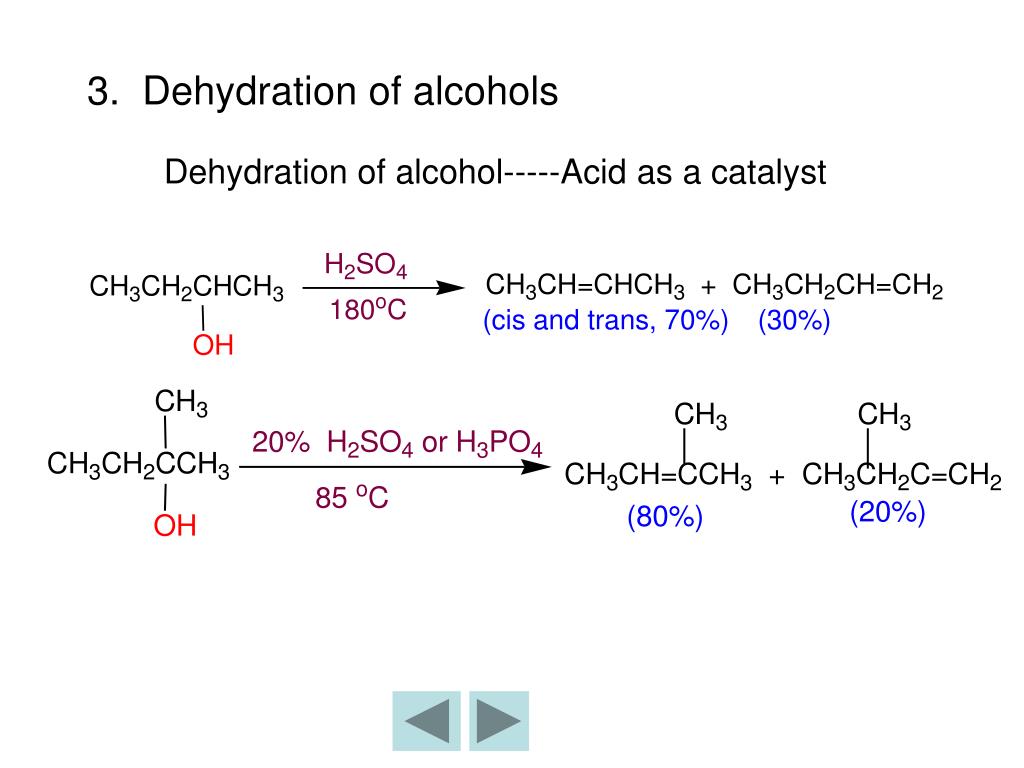
Dehydrating Agent Ethyl Alcohol . Leave the ethanol in the a large amount of drying agent. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. This mechanism is supported by the. Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. Ø commonly used dehydrating reagents are: The dehydration reaction of alcohols.
from www.slideserve.com
Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. The dehydration reaction of alcohols. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. This mechanism is supported by the. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. Ø commonly used dehydrating reagents are: Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Leave the ethanol in the a large amount of drying agent.
PPT Chapter 5 Alkenes PowerPoint Presentation, free download ID7013489
Dehydrating Agent Ethyl Alcohol Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. The dehydration reaction of alcohols. This mechanism is supported by the. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Ø commonly used dehydrating reagents are: Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. Leave the ethanol in the a large amount of drying agent. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c.
From www.biognost.com
Histanol 50 Biognost Dehydrating Agent Ethyl Alcohol One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Ø commonly used dehydrating reagents are: Leave the ethanol in the a large amount of drying agent. You can dry the ethanol by using drying agent such as molecular sieve (3a) or. Dehydrating Agent Ethyl Alcohol.
From www.2spi.com
Solvents and Dehydrating Agents SPI Supplies Dehydrating Agent Ethyl Alcohol Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. This mechanism is supported by the. Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. Leave the ethanol in the a large amount of drying agent. The dehydration reaction of alcohols. You can. Dehydrating Agent Ethyl Alcohol.
From readchemistry.com
Alkene Synthesis by Dehydration of Alcohols Read Chemistry Dehydrating Agent Ethyl Alcohol Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. This mechanism is supported by the. Leave the ethanol in the a large amount of drying agent.. Dehydrating Agent Ethyl Alcohol.
From ar.inspiredpencil.com
Dehydration Reaction Mechanism Of Alcohols Dehydrating Agent Ethyl Alcohol Ø commonly used dehydrating reagents are: Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. This mechanism is supported by the. Ethyl alcohol or ethanol. Dehydrating Agent Ethyl Alcohol.
From www.doubtnut.com
Give a mechanism for the acidcatalysed dehydration of ethyl alcohol Dehydrating Agent Ethyl Alcohol The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Leave the ethanol in the a large amount of drying agent. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. Ø commonly used dehydrating reagents are: Dehydration is achieved by placing the tissue in graded alcohols to avoid damage. Dehydrating Agent Ethyl Alcohol.
From www.youtube.com
Dehydration of Ethanol to Ethylene YouTube Dehydrating Agent Ethyl Alcohol You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. The dehydration reaction of alcohols. The most feasible explanation for the antimicrobial action of alcohol is denaturation. Dehydrating Agent Ethyl Alcohol.
From www.youtube.com
Dehydration of ethyl alcohol proceeds via formation of (A) carbonium Dehydrating Agent Ethyl Alcohol This mechanism is supported by the. Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Leave the ethanol in the a large amount of drying agent. Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. One way to synthesize alkenes is by. Dehydrating Agent Ethyl Alcohol.
From www.chemistrysteps.com
Alcohol Dehydration by E1 and E2 Elimination with Practice Problems Dehydrating Agent Ethyl Alcohol Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. One way to synthesize alkenes is by dehydration of alcohols, a process in. Dehydrating Agent Ethyl Alcohol.
From slideplayer.com
Hydrocarbons Molecules made of Hydrogen and Carbon ppt download Dehydrating Agent Ethyl Alcohol Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. You. Dehydrating Agent Ethyl Alcohol.
From sdjc123.en.made-in-china.com
Dehydrating Agent / CAS 67630 Ipa / Chemical Materials Isopropyl Dehydrating Agent Ethyl Alcohol The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. Dehydration is achieved. Dehydrating Agent Ethyl Alcohol.
From www.chemistrysteps.com
Alcohol Dehydration by E1 and E2 Elimination with Practice Problems Dehydrating Agent Ethyl Alcohol Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. The. Dehydrating Agent Ethyl Alcohol.
From www.coursehero.com
[Solved] Ethyl ether is made by the dehydration of ethyl alcohol Dehydrating Agent Ethyl Alcohol Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. The dehydration reaction of alcohols.. Dehydrating Agent Ethyl Alcohol.
From www.biognost.com
Dehydrating and rehydrating agents Archives Biognost Dehydrating Agent Ethyl Alcohol Ø commonly used dehydrating reagents are: Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Dehydrants (reagents used for dehydration). Dehydrating Agent Ethyl Alcohol.
From www.youtube.com
Dehydration of Alcohol to Ether Mechanism Ethers YouTube Dehydrating Agent Ethyl Alcohol This mechanism is supported by the. Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. The dehydration reaction of alcohols. Ø commonly used dehydrating reagents are: Ethyl alcohol or ethanol. Dehydrating Agent Ethyl Alcohol.
From www.polysciences.com
Ethanol Solution, 95 Polysciences, Inc. Dehydrating Agent Ethyl Alcohol You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose. Dehydrating Agent Ethyl Alcohol.
From chinafinechemicals.en.made-in-china.com
Dehydrating Agent Raw Material 99.9 Purity Isopropyl Alcohol Dehydrating Agent Ethyl Alcohol The dehydration reaction of alcohols. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. This mechanism is supported by the. Ø some reagents are just. Dehydrating Agent Ethyl Alcohol.
From www.doubtnut.com
Give a mechanism for the acidcatalysed dehydration of ethyl alcohol Dehydrating Agent Ethyl Alcohol Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. The dehydration reaction of alcohols. Dehydrants (reagents used for dehydration) ø many reagents are used for the. Dehydrating Agent Ethyl Alcohol.
From www.slideserve.com
PPT Dehydration of Alcohols to form Ethers PowerPoint Presentation Dehydrating Agent Ethyl Alcohol Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. Ø commonly used dehydrating reagents are: Leave the ethanol in the a large amount of drying agent. Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. The dehydration reaction of alcohols. Ethyl alcohol. Dehydrating Agent Ethyl Alcohol.
From sdarcticchem.en.made-in-china.com
Dehydrating Agent / CAS No. 67630 / Isopropyl Alcohol / Ipa China Dehydrating Agent Ethyl Alcohol Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Leave the ethanol in the a large amount of drying agent. Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. Ø commonly used dehydrating reagents are: Ø some reagents are just water removing. Dehydrating Agent Ethyl Alcohol.
From www.molecularsieveadsorbent.com
Dehydration Agent 4A Molecular Sieve Desiccant For Ethanol Ethylene Dehydrating Agent Ethyl Alcohol The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. This mechanism is supported by the. Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c.. Dehydrating Agent Ethyl Alcohol.
From chem.libretexts.org
Alkenes from Dehydration of Alcohols Chemistry LibreTexts Dehydrating Agent Ethyl Alcohol One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. This mechanism is supported by the. Dehydration is achieved by placing the tissue in graded alcohols. Dehydrating Agent Ethyl Alcohol.
From www.doubtnut.com
Write the mechanism of acid dehydration of ethanol to yield ethene. Dehydrating Agent Ethyl Alcohol Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Ø some reagents are just water removing agents, whereas some others are. Dehydrating Agent Ethyl Alcohol.
From icsechemistry16.blogspot.com
preparation of Ethene Dehydrating Agent Ethyl Alcohol Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. Ø commonly used dehydrating reagents are: The dehydration reaction of alcohols. Leave the ethanol in the a large amount of drying agent. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. Ethanol can be dehydrated to give ethene. Dehydrating Agent Ethyl Alcohol.
From www.mdsupplies.com
Akorn Pharmaceuticals Dehydrated Alcohol 98 215.00/EachPO6540 Dehydrating Agent Ethyl Alcohol The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. This mechanism. Dehydrating Agent Ethyl Alcohol.
From www.doubtnut.com
Give a mechanism for the acidcatalysed dehydration of ethyl alcohol Dehydrating Agent Ethyl Alcohol Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Leave the ethanol in the a large amount of drying agent. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. The dehydration reaction of alcohols. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of. Dehydrating Agent Ethyl Alcohol.
From www.researchgate.net
Equilibrium composition in ethanol dehydrogenation (A), in ethanol Dehydrating Agent Ethyl Alcohol You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Leave the ethanol in the a large amount of. Dehydrating Agent Ethyl Alcohol.
From www.youtube.com
H2SO4 as Powerful Dehydrating Agent PBlock Elements Chemistry Dehydrating Agent Ethyl Alcohol Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Leave the ethanol in the a large amount of drying agent. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is.. Dehydrating Agent Ethyl Alcohol.
From www.youtube.com
Give a mechanism for the acidcatalysed dehydration of ethyl alcohol to Dehydrating Agent Ethyl Alcohol Ø commonly used dehydrating reagents are: This mechanism is supported by the. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Dehydrants (reagents used for dehydration) ø many reagents are used for. Dehydrating Agent Ethyl Alcohol.
From www.youtube.com
Dehydration of ethyl alcohol with conc. H_(2)SO_(4) at 413 K gives Dehydrating Agent Ethyl Alcohol Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. Ø commonly used dehydrating reagents are: Ethanol can be dehydrated. Dehydrating Agent Ethyl Alcohol.
From www.slideserve.com
PPT Reactions of Alcohols PowerPoint Presentation, free download ID Dehydrating Agent Ethyl Alcohol Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. This mechanism is supported by the. Ø commonly used dehydrating reagents are: Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. You can dry the ethanol by using drying agent such as molecular. Dehydrating Agent Ethyl Alcohol.
From aneskey.com
Chemical Neurolysis Anesthesia Key Dehydrating Agent Ethyl Alcohol Ø commonly used dehydrating reagents are: Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. Ø some reagents are just water removing agents, whereas some others are both water removing. Dehydrating Agent Ethyl Alcohol.
From studylib.net
the dehydration of alcohols Dehydrating Agent Ethyl Alcohol Ø commonly used dehydrating reagents are: One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. The dehydration reaction of alcohols. Ø some reagents are just water removing agents, whereas some others are both water removing and suitable for subsequent processing. Leave. Dehydrating Agent Ethyl Alcohol.
From www.slideserve.com
PPT Chapter 5 Alkenes PowerPoint Presentation, free download ID7013489 Dehydrating Agent Ethyl Alcohol This mechanism is supported by the. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration of plant specimens. One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo e1 or e2 mechanisms to lose water and form a double bond. Leave the ethanol in the a large amount of drying. Dehydrating Agent Ethyl Alcohol.
From www.doubtnut.com
Write the mechanism of acid catalyzed dehydration of ethanol t Dehydrating Agent Ethyl Alcohol Dehydration is achieved by placing the tissue in graded alcohols to avoid damage to the tissues. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. The dehydration reaction of alcohols. Leave the ethanol in the a large amount of drying agent. Dehydrants (reagents used for dehydration) ø many reagents are used for the dehydration. Dehydrating Agent Ethyl Alcohol.
From www.numerade.com
SOLVED O3 Ethyl ether is made by dehydration of ethyl alcohol in the Dehydrating Agent Ethyl Alcohol Ethanol can be dehydrated to give ethene by heating it with an excess of concentrated sulphuric acid at about 170°c. Ethyl alcohol or ethanol is a clear, colorless, inflammable liquid with a pleasant odor, is. You can dry the ethanol by using drying agent such as molecular sieve (3a) or by using anhydrous calcium sulfate or magnesium sulfate. Ø commonly. Dehydrating Agent Ethyl Alcohol.