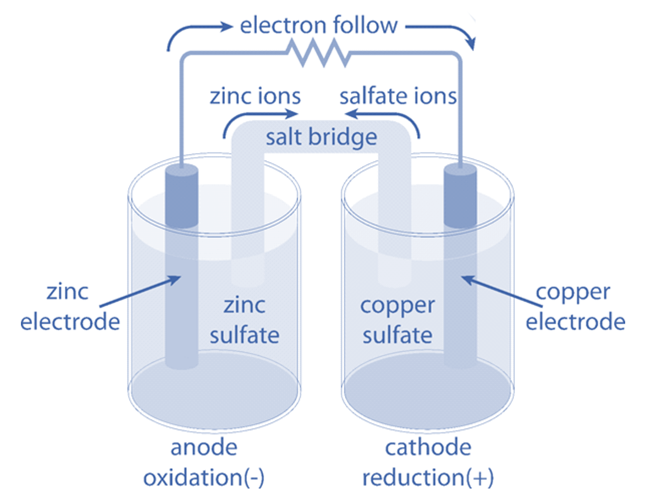
Galvanic Vs Voltaic . A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. In doing so, a voltaic/ galvanic cell is created. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Nonspontaneous reactions occur in electrolytic cells. What is the difference between voltaic or galvanic cells and electrolytic cells? There are two types of electrochemical cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Spontaneous reactions occur in galvanic (voltaic) cells;
from scienceinfo.com
The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Electrochemical cells have two conductive electrodes, called the anode and the cathode. In doing so, a voltaic/ galvanic cell is created. There are two types of electrochemical cells. Nonspontaneous reactions occur in electrolytic cells. What is the difference between voltaic or galvanic cells and electrolytic cells? Spontaneous reactions occur in galvanic (voltaic) cells;
Galvanic Cell (Voltaic Cell) Definition, Working Principle
Galvanic Vs Voltaic In doing so, a voltaic/ galvanic cell is created. What is the difference between voltaic or galvanic cells and electrolytic cells? The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Spontaneous reactions occur in galvanic (voltaic) cells; In doing so, a voltaic/ galvanic cell is created. There are two types of electrochemical cells. Nonspontaneous reactions occur in electrolytic cells. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an.
From in.pinterest.com
three different types of electronic devices are shown in this diagram Galvanic Vs Voltaic A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Spontaneous reactions occur in galvanic (voltaic) cells; In doing so, a voltaic/ galvanic cell is created. There are two types of electrochemical cells. The reactions are put into two different containers and a wire is used to drive the electrons from one. Galvanic Vs Voltaic.
From www.studypool.com
SOLUTION Introduction to Galvanic Cells vs Voltaic Cells Studypool Galvanic Vs Voltaic The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Spontaneous reactions occur in galvanic (voltaic) cells; Nonspontaneous reactions occur in electrolytic cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. In doing so, a voltaic/ galvanic cell is created. A galvanic (voltaic). Galvanic Vs Voltaic.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download Galvanic Vs Voltaic Electrochemical cells have two conductive electrodes, called the anode and the cathode. What is the difference between voltaic or galvanic cells and electrolytic cells? In doing so, a voltaic/ galvanic cell is created. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Spontaneous reactions occur in. Galvanic Vs Voltaic.
From www.youtube.com
Redox Electrolytic vs. Galvanic/Voltaic Cells YouTube Galvanic Vs Voltaic In doing so, a voltaic/ galvanic cell is created. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Nonspontaneous reactions occur in electrolytic cells. There are two types of electrochemical cells. A galvanic (voltaic). Galvanic Vs Voltaic.
From www.youtube.com
ELECTROLYTIC CELL Vs VOLTAIC (GALVANIC) CELL YouTube Galvanic Vs Voltaic What is the difference between voltaic or galvanic cells and electrolytic cells? There are two types of electrochemical cells. Nonspontaneous reactions occur in electrolytic cells. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Spontaneous reactions occur in galvanic (voltaic) cells; Electrochemical cells have two conductive. Galvanic Vs Voltaic.
From www.vrogue.co
Voltaic Vs Galvanic Cell vrogue.co Galvanic Vs Voltaic There are two types of electrochemical cells. In doing so, a voltaic/ galvanic cell is created. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. What is the difference between voltaic or galvanic cells and electrolytic cells? The reactions are put into two different containers and a wire is used to. Galvanic Vs Voltaic.
From www.youtube.com
The differences between the voltaic cell and the electrolytic cell Galvanic Vs Voltaic What is the difference between voltaic or galvanic cells and electrolytic cells? Electrochemical cells have two conductive electrodes, called the anode and the cathode. Nonspontaneous reactions occur in electrolytic cells. Spontaneous reactions occur in galvanic (voltaic) cells; A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. There are two types of. Galvanic Vs Voltaic.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Galvanic Vs Voltaic What is the difference between voltaic or galvanic cells and electrolytic cells? Electrochemical cells have two conductive electrodes, called the anode and the cathode. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Nonspontaneous reactions occur in electrolytic cells. Spontaneous reactions occur in galvanic (voltaic) cells; The reactions are put into. Galvanic Vs Voltaic.
From mavink.com
Voltaic Vs Galvanic Cell Galvanic Vs Voltaic Spontaneous reactions occur in galvanic (voltaic) cells; Nonspontaneous reactions occur in electrolytic cells. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. There are two types of electrochemical cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The reactions are put into two different containers and a. Galvanic Vs Voltaic.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube Galvanic Vs Voltaic In doing so, a voltaic/ galvanic cell is created. Spontaneous reactions occur in galvanic (voltaic) cells; A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Nonspontaneous reactions occur in electrolytic cells. The reactions are put into two different containers and a wire is used to drive the electrons from one side. Galvanic Vs Voltaic.
From stock.adobe.com
Electrochemical cell diagram. Galvanic cell or voltaic cell. Zinc anode Galvanic Vs Voltaic A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Nonspontaneous reactions occur in electrolytic cells. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. What is the difference between voltaic or galvanic cells and electrolytic cells? In. Galvanic Vs Voltaic.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts Galvanic Vs Voltaic Electrochemical cells have two conductive electrodes, called the anode and the cathode. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Nonspontaneous reactions occur in electrolytic cells. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. In. Galvanic Vs Voltaic.
From lavelle.chem.ucla.edu
Galvanic vs Voltaic Cells CHEMISTRY COMMUNITY Galvanic Vs Voltaic A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. In doing so, a voltaic/ galvanic cell is created. What is the difference between voltaic or galvanic cells and electrolytic cells? Spontaneous reactions occur in galvanic (voltaic) cells; There are two types of electrochemical cells. Electrochemical cells have two conductive electrodes, called. Galvanic Vs Voltaic.
From www.youtube.com
MCAT Question of the Day Galvanic and Voltaic Cells YouTube Galvanic Vs Voltaic What is the difference between voltaic or galvanic cells and electrolytic cells? Electrochemical cells have two conductive electrodes, called the anode and the cathode. Spontaneous reactions occur in galvanic (voltaic) cells; The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. There are two types of electrochemical. Galvanic Vs Voltaic.
From www.youtube.com
Electrochemistry Galvanic/Voltaic vs. Electrolytic Cell YouTube Galvanic Vs Voltaic Spontaneous reactions occur in galvanic (voltaic) cells; In doing so, a voltaic/ galvanic cell is created. Electrochemical cells have two conductive electrodes, called the anode and the cathode. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. There are two types of electrochemical cells. Nonspontaneous reactions occur in electrolytic cells. What. Galvanic Vs Voltaic.
From vdocuments.mx
1 Galvanic cells Electrochemical cells fall into one of two basic types Galvanic Vs Voltaic Nonspontaneous reactions occur in electrolytic cells. What is the difference between voltaic or galvanic cells and electrolytic cells? In doing so, a voltaic/ galvanic cell is created. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Spontaneous reactions occur in galvanic (voltaic) cells; Electrochemical cells have two conductive electrodes, called the. Galvanic Vs Voltaic.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Galvanic Vs Voltaic In doing so, a voltaic/ galvanic cell is created. There are two types of electrochemical cells. Spontaneous reactions occur in galvanic (voltaic) cells; A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. The reactions are put into two different containers and a wire is used to drive the electrons from one. Galvanic Vs Voltaic.
From stock.adobe.com
A galvanic cell or voltaic cell, is an electrochemical cell in which an Galvanic Vs Voltaic A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Spontaneous reactions occur in galvanic (voltaic) cells; Nonspontaneous reactions occur in electrolytic cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. What is the difference between voltaic or galvanic cells and electrolytic cells? There are two types of. Galvanic Vs Voltaic.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Galvanic Vs Voltaic Electrochemical cells have two conductive electrodes, called the anode and the cathode. There are two types of electrochemical cells. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. What is the difference between voltaic or galvanic cells and electrolytic cells? The reactions are put into two different containers and a wire. Galvanic Vs Voltaic.
From sklep.foteks.pl
Fototapeta Vector scientific illustration of the electrolysis processes Galvanic Vs Voltaic What is the difference between voltaic or galvanic cells and electrolytic cells? Nonspontaneous reactions occur in electrolytic cells. There are two types of electrochemical cells. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The reactions are put into. Galvanic Vs Voltaic.
From www.youtube.com
Introduction to Galvanic Cells & Voltaic Cells YouTube Galvanic Vs Voltaic Electrochemical cells have two conductive electrodes, called the anode and the cathode. Nonspontaneous reactions occur in electrolytic cells. What is the difference between voltaic or galvanic cells and electrolytic cells? The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Spontaneous reactions occur in galvanic (voltaic) cells;. Galvanic Vs Voltaic.
From www.youtube.com
Galvanic cell or Voltaic cell Definition ,Construction and Working Galvanic Vs Voltaic A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. In doing so, a voltaic/ galvanic cell is created. Spontaneous reactions occur in galvanic (voltaic) cells; The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Nonspontaneous reactions occur. Galvanic Vs Voltaic.
From www.shutterstock.com
Voltaic Galvanic Cell Copper Cathode Magnesium Stock Vector (Royalty Galvanic Vs Voltaic What is the difference between voltaic or galvanic cells and electrolytic cells? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Nonspontaneous reactions occur in electrolytic cells. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. In. Galvanic Vs Voltaic.
From leah4sci.com
Electrochemistry Galvanic / Voltaic and Electrolytic Cells MCAT and Galvanic Vs Voltaic In doing so, a voltaic/ galvanic cell is created. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Spontaneous reactions occur in galvanic (voltaic) cells; A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Nonspontaneous reactions occur. Galvanic Vs Voltaic.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Galvanic Vs Voltaic A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Nonspontaneous reactions occur in electrolytic cells. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. What. Galvanic Vs Voltaic.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Galvanic Vs Voltaic A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Nonspontaneous reactions occur in electrolytic cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. What is the difference between voltaic or galvanic cells and electrolytic cells? The reactions are put into two different containers and a wire is. Galvanic Vs Voltaic.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Galvanic Vs Voltaic Electrochemical cells have two conductive electrodes, called the anode and the cathode. Nonspontaneous reactions occur in electrolytic cells. In doing so, a voltaic/ galvanic cell is created. What is the difference between voltaic or galvanic cells and electrolytic cells? There are two types of electrochemical cells. Spontaneous reactions occur in galvanic (voltaic) cells; A galvanic (voltaic) cell uses the energy. Galvanic Vs Voltaic.
From stock.adobe.com
A galvanic cell or voltaic cell, named after the scientists Luigi Galvanic Vs Voltaic In doing so, a voltaic/ galvanic cell is created. Nonspontaneous reactions occur in electrolytic cells. What is the difference between voltaic or galvanic cells and electrolytic cells? Electrochemical cells have two conductive electrodes, called the anode and the cathode. Spontaneous reactions occur in galvanic (voltaic) cells; A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to. Galvanic Vs Voltaic.
From www.youtube.com
How To Draw Galvanic Cells and Voltaic Cells Electrochemistry YouTube Galvanic Vs Voltaic The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. Spontaneous reactions occur in galvanic (voltaic) cells; In doing so, a voltaic/ galvanic cell is created. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. Nonspontaneous reactions occur. Galvanic Vs Voltaic.
From scienceinfo.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Galvanic Vs Voltaic Electrochemical cells have two conductive electrodes, called the anode and the cathode. Nonspontaneous reactions occur in electrolytic cells. Spontaneous reactions occur in galvanic (voltaic) cells; There are two types of electrochemical cells. In doing so, a voltaic/ galvanic cell is created. The reactions are put into two different containers and a wire is used to drive the electrons from one. Galvanic Vs Voltaic.
From www.youtube.com
galvanic cell or v Voltaic cell YouTube Galvanic Vs Voltaic There are two types of electrochemical cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Nonspontaneous reactions occur in electrolytic cells. Spontaneous reactions occur in galvanic (voltaic) cells; What is the difference between voltaic or galvanic cells and electrolytic cells? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. Galvanic Vs Voltaic.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Galvanic Vs Voltaic Nonspontaneous reactions occur in electrolytic cells. Spontaneous reactions occur in galvanic (voltaic) cells; In doing so, a voltaic/ galvanic cell is created. Electrochemical cells have two conductive electrodes, called the anode and the cathode. What is the difference between voltaic or galvanic cells and electrolytic cells? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to. Galvanic Vs Voltaic.
From mavink.com
Voltaic Vs Galvanic Cell Galvanic Vs Voltaic What is the difference between voltaic or galvanic cells and electrolytic cells? Nonspontaneous reactions occur in electrolytic cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. There are two types of electrochemical cells. In doing so, a voltaic/. Galvanic Vs Voltaic.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner Galvanic Vs Voltaic There are two types of electrochemical cells. Nonspontaneous reactions occur in electrolytic cells. In doing so, a voltaic/ galvanic cell is created. Electrochemical cells have two conductive electrodes, called the anode and the cathode. What is the difference between voltaic or galvanic cells and electrolytic cells? The reactions are put into two different containers and a wire is used to. Galvanic Vs Voltaic.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Galvanic Vs Voltaic Electrochemical cells have two conductive electrodes, called the anode and the cathode. What is the difference between voltaic or galvanic cells and electrolytic cells? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. There are two types of electrochemical cells. Spontaneous reactions occur in galvanic (voltaic) cells; Nonspontaneous reactions occur in. Galvanic Vs Voltaic.