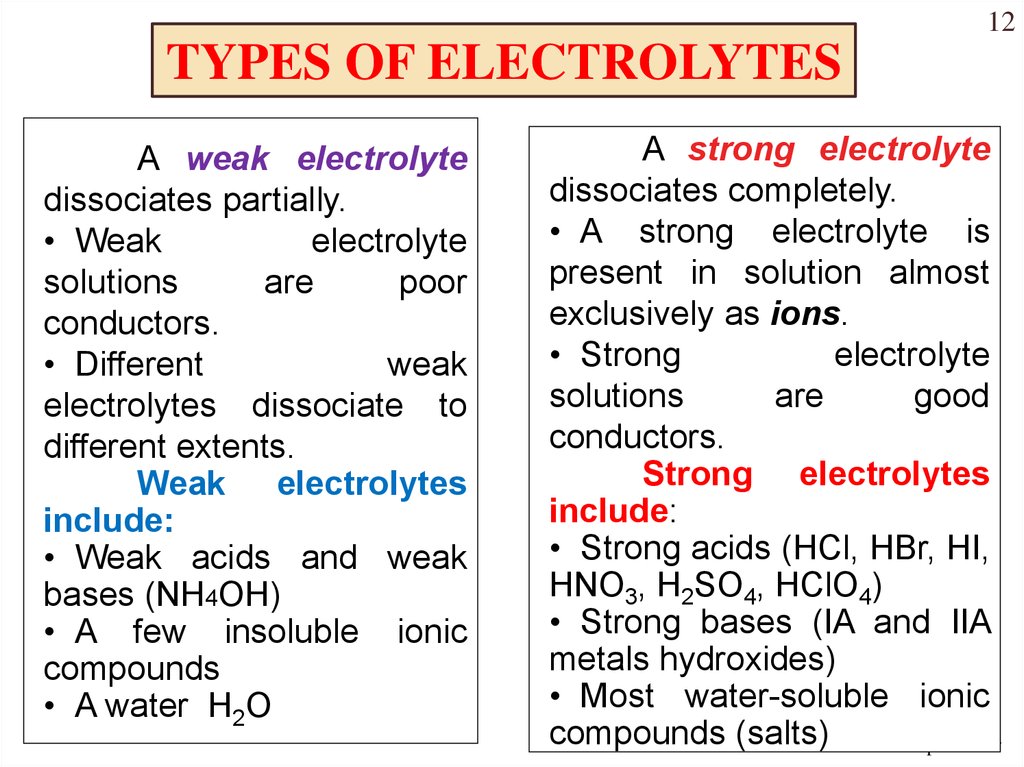
Example Of Chemical Compound Electrolyte . electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. In order to conduct a. Electrolytes may be covalent compounds that chemically. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are examples of minerals. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. substances that dissolve in water to yield ions are called electrolytes. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water.
from en.ppt-online.org
the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. In order to conduct a. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. substances that dissolve in water to yield ions are called electrolytes. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. Electrolytes may be covalent compounds that chemically. electrolytes are examples of minerals. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc.
Aqueous Solutions of Electrolytes online presentation
Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. substances that dissolve in water to yield ions are called electrolytes. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. electrolytes are examples of minerals. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. Electrolytes may be covalent compounds that chemically. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. In order to conduct a.
From www.freepik.com
Free Vector Electrolysis diagram experiment for education Example Of Chemical Compound Electrolyte electrolytes are examples of minerals. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. substances that dissolve in water to yield ions are called electrolytes. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. the most familiar electrolytes are acids,. Example Of Chemical Compound Electrolyte.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID2706781 Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. electrolytes are examples of. Example Of Chemical Compound Electrolyte.
From courses.lumenlearning.com
Electrolysis Chemistry Example Of Chemical Compound Electrolyte electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. Electrolytes may be covalent compounds that chemically. substances that dissolve in water to yield ions are called electrolytes. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. the most familiar electrolytes are acids, bases,. Example Of Chemical Compound Electrolyte.
From www.scribd.com
Electrolytes Electrolyte Chemical Compounds Example Of Chemical Compound Electrolyte for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. In order to conduct a. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are examples of minerals. electrolytes are. Example Of Chemical Compound Electrolyte.
From www.youtube.com
Electrolytes Definition, Examples, & Practice YouTube Example Of Chemical Compound Electrolyte In order to conduct a. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. Electrolytes may be covalent compounds that chemically. substances that dissolve in water to yield ions are called electrolytes. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. electrolytes are examples of minerals. the most familiar electrolytes are acids,. Example Of Chemical Compound Electrolyte.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Example Of Chemical Compound Electrolyte electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are examples of minerals. substances that dissolve in water to yield ions are called electrolytes. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. . Example Of Chemical Compound Electrolyte.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Example Of Chemical Compound Electrolyte electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. In order to conduct a. Electrolytes may be covalent compounds that chemically. electrolytes are examples of minerals. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc.. Example Of Chemical Compound Electrolyte.
From www.slideserve.com
PPT Chemical reactions PowerPoint Presentation, free download ID Example Of Chemical Compound Electrolyte an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. In order to conduct a. electrolytes are examples of minerals. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. substances that dissolve in water to yield. Example Of Chemical Compound Electrolyte.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Example Of Chemical Compound Electrolyte an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. substances that dissolve in water to yield ions are called electrolytes. electrolytes are examples of minerals. for example, \(\ce{nacl}\), \(\ce{hno3}\),. Example Of Chemical Compound Electrolyte.
From healthjade.com
Electrolytes Sources and Foods High In Electrolytes Example Of Chemical Compound Electrolyte electrolytes are examples of minerals. substances that dissolve in water to yield ions are called electrolytes. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. Electrolytes may be covalent compounds that chemically. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents. Example Of Chemical Compound Electrolyte.
From www.ck12.org
Solvation, Dissociation, and Electrolytes Example 4 ( Video Example Of Chemical Compound Electrolyte an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. electrolytes are examples of minerals. Electrolytes may be covalent compounds that chemically. an ionic compound for example, sodium chloride dissolved in water is. Example Of Chemical Compound Electrolyte.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Example Of Chemical Compound Electrolyte Electrolytes may be covalent compounds that chemically. electrolytes are examples of minerals. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are chemicals that break into ions (ionize). Example Of Chemical Compound Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Example Of Chemical Compound Electrolyte electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. electrolytes are examples of minerals. Electrolytes. Example Of Chemical Compound Electrolyte.
From slideplayer.com
Chapter 3 Chemical Compounds. ppt download Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. In order to conduct a. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. for example, \(\ce{nacl}\),. Example Of Chemical Compound Electrolyte.
From www.w3schools.blog
Strong and weak electrolytes W3schools Example Of Chemical Compound Electrolyte Electrolytes may be covalent compounds that chemically. electrolytes are examples of minerals. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. In order to conduct a. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. . Example Of Chemical Compound Electrolyte.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. electrolytes are examples of minerals. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. an ionic compound for example, sodium chloride. Example Of Chemical Compound Electrolyte.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Example Of Chemical Compound Electrolyte electrolytes are examples of minerals. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. In order to conduct a. Electrolytes may be covalent compounds that chemically. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. for example, \(\ce{nacl}\), \(\ce{hno3}\),. Example Of Chemical Compound Electrolyte.
From app.pandai.org
Electrolytic Cell Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. In order to conduct a. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\),. Example Of Chemical Compound Electrolyte.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download Example Of Chemical Compound Electrolyte electrolytes are examples of minerals. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. Electrolytes may be covalent compounds that chemically. In order to conduct a. an electrolyte is a compound that conducts an. Example Of Chemical Compound Electrolyte.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. Electrolytes may be covalent compounds that chemically. electrolytes are examples of minerals. an. Example Of Chemical Compound Electrolyte.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Example Of Chemical Compound Electrolyte an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. substances that dissolve in water to yield ions are called electrolytes. In order to conduct a. electrolytes are examples of. Example Of Chemical Compound Electrolyte.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Example Of Chemical Compound Electrolyte an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. In order to conduct a. substances that dissolve in water to yield ions are called electrolytes. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. Electrolytes may be covalent compounds. Example Of Chemical Compound Electrolyte.
From www.animalia-life.club
Electrolyte Chemistry Example Of Chemical Compound Electrolyte the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. substances that dissolve in water to yield ions are called electrolytes. electrolytes are examples of minerals. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte. Example Of Chemical Compound Electrolyte.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Example Of Chemical Compound Electrolyte the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. substances that dissolve in water to yield ions are called electrolytes. electrolytes are examples of minerals. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are chemicals that. Example Of Chemical Compound Electrolyte.
From www.kentchemistry.com
Electrolytes Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. electrolytes are chemicals that break into ions (ionize) when they. Example Of Chemical Compound Electrolyte.
From slideplayer.com
Chapter 3 Chemical Compounds. ppt download Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. for. Example Of Chemical Compound Electrolyte.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Example Of Chemical Compound Electrolyte the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. electrolytes are examples of minerals. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. an electrolyte is a compound that conducts an. Example Of Chemical Compound Electrolyte.
From www.thoughtco.com
Chemistry Examples Strong and Weak Electrolytes Example Of Chemical Compound Electrolyte an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. In order to conduct a. electrolytes are examples of minerals. substances that dissolve in water to yield ions are called electrolytes. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it. Example Of Chemical Compound Electrolyte.
From www.animalia-life.club
Electrolyte Chemistry Example Of Chemical Compound Electrolyte an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. substances that dissolve in water to yield ions are called electrolytes. In order to conduct a. electrolytes are examples of minerals.. Example Of Chemical Compound Electrolyte.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Example Of Chemical Compound Electrolyte electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts.. Example Of Chemical Compound Electrolyte.
From in.pinterest.com
What is Electrolysis and Electrolyte with examples. Science fair Example Of Chemical Compound Electrolyte the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically. electrolytes are chemicals that break into ions (ionize) when they are dissolved in water. an ionic compound for example, sodium. Example Of Chemical Compound Electrolyte.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. electrolytes are examples of minerals. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such. Example Of Chemical Compound Electrolyte.
From www.slideserve.com
PPT Chemical compounds PowerPoint Presentation, free download ID Example Of Chemical Compound Electrolyte electrolytes are examples of minerals. substances that dissolve in water to yield ions are called electrolytes. In order to conduct a. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. Electrolytes may be covalent compounds that chemically. an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. electrolytes. Example Of Chemical Compound Electrolyte.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID181760 Example Of Chemical Compound Electrolyte an electrolyte is a compound that conducts an electric current when it is in an aqueous solution or melted. for example, \(\ce{nacl}\), \(\ce{hno3}\), \(\ce{hclo3}\), \(\ce{cacl2}\) etc. substances that dissolve in water to yield ions are called electrolytes. the most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. . Example Of Chemical Compound Electrolyte.
From www.youtube.com
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes Example Of Chemical Compound Electrolyte substances that dissolve in water to yield ions are called electrolytes. In order to conduct a. an ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts. electrolytes are examples of minerals. Electrolytes may be covalent compounds that chemically. an electrolyte is a compound that conducts an electric current when. Example Of Chemical Compound Electrolyte.