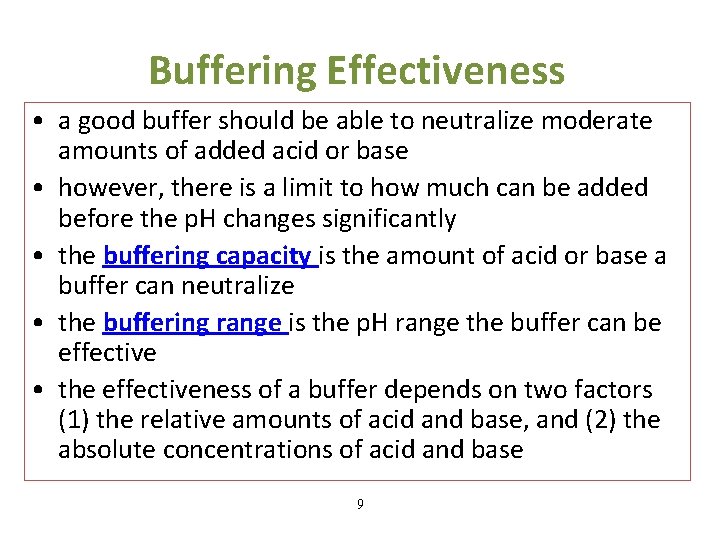
What Makes A Buffer Effective . Calculate the amounts of acid and base needed to prepare a buffer of a given ph. A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the ph range over which they. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Many things, such as changes in. Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. This turns out to be. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. As a general rule of thumb, the relative amounts. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.
from slidetodoc.com
Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. Many things, such as changes in. This turns out to be. A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. As a general rule of thumb, the relative amounts. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents).
Introduction to Buffers These solutions contain relatively high
What Makes A Buffer Effective As a general rule of thumb, the relative amounts. Many things, such as changes in. Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. As a general rule of thumb, the relative amounts. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. This turns out to be. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. Buffers are characterized by the ph range over which they.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Makes A Buffer Effective A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Many things, such as changes in.. What Makes A Buffer Effective.
From www.slideserve.com
PPT All About Buffers PowerPoint Presentation, free download ID1321295 What Makes A Buffer Effective Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. Buffers are characterized by the ph range over which they. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers resist dramatic changes in ph by being composed of certain. What Makes A Buffer Effective.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Makes A Buffer Effective This turns out to be. The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Buffers function best when the pk a of the conjugate weak acid used is close to. What Makes A Buffer Effective.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID1149749 What Makes A Buffer Effective This turns out to be. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffers are characterized by the ph range over which they. Buffers should be very soluble in water,. What Makes A Buffer Effective.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Makes A Buffer Effective A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: As a general rule of thumb, the relative amounts. Calculate the amounts. What Makes A Buffer Effective.
From hillalifeller.blogspot.com
What Makes A Good Buffer System Hill Alifeller What Makes A Buffer Effective This turns out to be. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. As a general rule of thumb, the relative amounts. Many things, such as changes in. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers function best when the pk a of the. What Makes A Buffer Effective.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid What Makes A Buffer Effective As a general rule of thumb, the relative amounts. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers should be very. What Makes A Buffer Effective.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Makes A Buffer Effective As a general rule of thumb, the relative amounts. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. This turns out to be. The effective buffering range of a buffer. What Makes A Buffer Effective.
From www.youtube.com
BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS What Makes A Buffer Effective The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. Calculate the amounts of acid and base needed to prepare a buffer. What Makes A Buffer Effective.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Makes A Buffer Effective Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both. What Makes A Buffer Effective.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Makes A Buffer Effective Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). As a general rule of thumb, the relative amounts. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers function best when the pk a of the conjugate weak acid used is close to the. What Makes A Buffer Effective.
From www.slideshare.net
Ch 18 buffers What Makes A Buffer Effective The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). A. What Makes A Buffer Effective.
From slidetodoc.com
PREPARATION OF BUFFERS To make an effective buffer What Makes A Buffer Effective As a general rule of thumb, the relative amounts. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers are characterized by the ph range over which they. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers resist dramatic changes in ph by being composed of. What Makes A Buffer Effective.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 What Makes A Buffer Effective A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the ph range over which they. The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. Buffers function best. What Makes A Buffer Effective.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Makes A Buffer Effective Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Many things, such as changes in. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. What Makes A Buffer Effective.
From www.promegaconnections.com
What Makes a "Good" Buffer? What Makes A Buffer Effective This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Many things, such as changes in. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffers are characterized by the ph range over which they. Either a weak acid plus a salt derived from that weak acid, or. What Makes A Buffer Effective.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID What Makes A Buffer Effective Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). Many things, such. What Makes A Buffer Effective.
From slideplayer.com
Buffer Effectiveness, Titrations, and pH Curves ppt download What Makes A Buffer Effective A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. As a general rule of thumb, the relative amounts. This turns out to be. Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). Buffers resist dramatic changes in ph. What Makes A Buffer Effective.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Makes A Buffer Effective A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the. What Makes A Buffer Effective.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Makes A Buffer Effective A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. As a general rule of thumb, the relative amounts. The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. Either a weak. What Makes A Buffer Effective.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Makes A Buffer Effective Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers are. What Makes A Buffer Effective.
From slidetodoc.com
PREPARATION OF BUFFERS To make an effective buffer What Makes A Buffer Effective Buffers should be very soluble in water, and minimally so in nonpolar solvents (fats, oils, and organic solvents). Buffers are characterized by the ph range over which they. This turns out to be. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is able to. What Makes A Buffer Effective.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Makes A Buffer Effective Calculate the amounts of acid and base needed to prepare a buffer of a given ph. A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or. What Makes A Buffer Effective.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Makes A Buffer Effective Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. As a general rule of thumb, the relative amounts. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. This characteristic makes buffers important in biological and chemical applications where ph. What Makes A Buffer Effective.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Makes A Buffer Effective As a general rule of thumb, the relative amounts. Many things, such as changes in. A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. What Makes A Buffer Effective.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint What Makes A Buffer Effective Many things, such as changes in. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Either a weak acid plus a salt derived from that weak acid, or a weak base. What Makes A Buffer Effective.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Makes A Buffer Effective This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Many things, such as changes in. Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. As a general rule of thumb, the relative amounts. A buffer is a solution that maintains. What Makes A Buffer Effective.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Makes A Buffer Effective A buffer is able to resist ph change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts. The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. This characteristic makes buffers important in biological and chemical applications where ph. What Makes A Buffer Effective.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID2814082 What Makes A Buffer Effective Calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that. What Makes A Buffer Effective.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Makes A Buffer Effective Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. A buffer is able to resist ph change. What Makes A Buffer Effective.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Makes A Buffer Effective This turns out to be. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. As a general rule of thumb, the relative amounts. Buffers resist dramatic changes in ph by being. What Makes A Buffer Effective.
From courses.lumenlearning.com
Buffers Chemistry for Majors What Makes A Buffer Effective This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. A buffer is most effective when the amounts of acid and conjugate base are approximately equal. Buffers are characterized by. What Makes A Buffer Effective.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Makes A Buffer Effective Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. Buffers are characterized by the ph range over which they. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Many things, such as changes in. Either a weak acid plus a salt. What Makes A Buffer Effective.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Makes A Buffer Effective Calculate the amounts of acid and base needed to prepare a buffer of a given ph. The effective buffering range of a buffer is a factor of its pk a, the dissociation constant of the weak acid in the buffering system. Many things, such as changes in. As a general rule of thumb, the relative amounts. A buffer is able. What Makes A Buffer Effective.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation What Makes A Buffer Effective Buffers are characterized by the ph range over which they. Buffers function best when the pk a of the conjugate weak acid used is close to the desired working range of the buffer. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: This turns out to be. Many things, such as changes in. A buffer. What Makes A Buffer Effective.