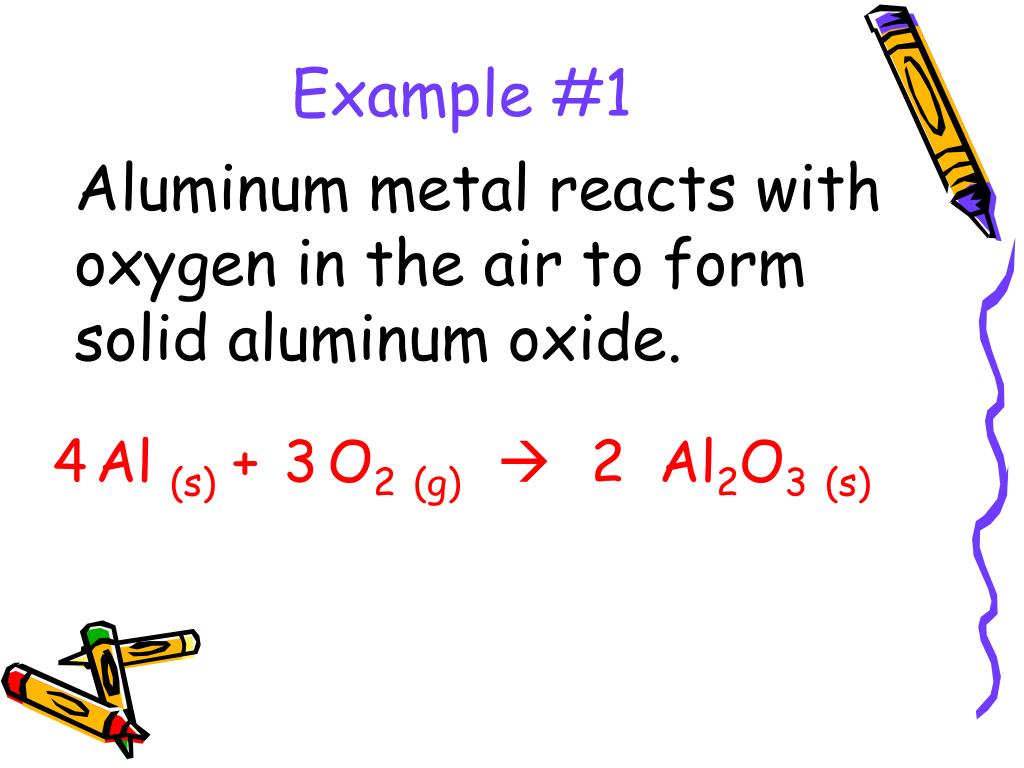
Aluminum Oxide Yields Aluminum And Oxygen . When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. In both cases, the metal acquires a positive charge by transferring. This is the unbalanced equation described. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Al + o2 → al2o3. Aluminum oxide is formed when aluminum reacts with oxygen. Achieve a common 6 oxygen atoms on either side of the. Your solution’s ready to go! In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Since metallic aluminum is very reactive with atmospheric. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Aluminium burns in oxygen to form al2o3.
from www.slideserve.com
Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. This is the unbalanced equation described. Your solution’s ready to go! In both cases, the metal acquires a positive charge by transferring. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Aluminum oxide is formed when aluminum reacts with oxygen. Since metallic aluminum is very reactive with atmospheric. Al + o2 → al2o3. Aluminum oxide is responsible for resistance of metallic aluminum to weathering.
PPT Unit 8 Chemical Reactions PowerPoint Presentation, free download
Aluminum Oxide Yields Aluminum And Oxygen This is the unbalanced equation described. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Aluminum oxide is formed when aluminum reacts with oxygen. Aluminium burns in oxygen to form al2o3. In both cases, the metal acquires a positive charge by transferring. Since metallic aluminum is very reactive with atmospheric. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. Achieve a common 6 oxygen atoms on either side of the. Al + o2 → al2o3. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Your solution’s ready to go! This is the unbalanced equation described.
From www.echemi.com
Aluminium oxide phase diagram ECHEMI Aluminum Oxide Yields Aluminum And Oxygen Aluminum oxide is formed when aluminum reacts with oxygen. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Since metallic aluminum is very reactive with atmospheric. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. Your solution’s ready to go! Aluminium burns in oxygen to form al2o3. Al. Aluminum Oxide Yields Aluminum And Oxygen.
From www.alamy.com
aluminum oxide or alumina, chemical compound of aluminum and oxygen Aluminum Oxide Yields Aluminum And Oxygen Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Aluminium burns in oxygen to form al2o3. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. This is the unbalanced equation described. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability.. Aluminum Oxide Yields Aluminum And Oxygen.
From www.quanswer.com
When 21.6g of aluminum reacts with oxygen, 20.8g of aluminum oxide is Aluminum Oxide Yields Aluminum And Oxygen Achieve a common 6 oxygen atoms on either side of the. Aluminium burns in oxygen to form al2o3. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Your solution’s ready to go! Since metallic aluminum is very reactive with atmospheric. Aluminum oxide is formed when aluminum reacts with oxygen. When exposed to air, aluminum metal develops a continuous,. Aluminum Oxide Yields Aluminum And Oxygen.
From www.bartleby.com
Answered Solid aluminum and gaseous oxygen react… bartleby Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Aluminum oxide is formed when aluminum reacts with oxygen. Since metallic aluminum is very. Aluminum Oxide Yields Aluminum And Oxygen.
From stock.adobe.com
Thin layer of aluminium oxide makes the appearance of aluminium metal Aluminum Oxide Yields Aluminum And Oxygen Al + o2 → al2o3. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Since metallic aluminum is very reactive with atmospheric. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Aluminum and oxygen react to form aluminum oxide by the equation.what. Aluminum Oxide Yields Aluminum And Oxygen.
From www.coursehero.com
[Solved] Aluminum and oxygen react to form aluminum oxide. How many Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! Al + o2 → al2o3. Aluminum oxide is formed when aluminum reacts with oxygen. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. This is the unbalanced equation described. In both cases, the metal acquires a positive charge by transferring. Aluminum. Aluminum Oxide Yields Aluminum And Oxygen.
From www.numerade.com
SOLVED What is the name of a compound having the formula Al2O3? (5 Aluminum Oxide Yields Aluminum And Oxygen In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Achieve a common 6 oxygen atoms on either side of the. Aluminum and oxygen react to form aluminum oxide by the equation.what is the. Aluminum Oxide Yields Aluminum And Oxygen.
From bernard-blogrose.blogspot.com
Aluminum Reacts With Oxygen to Produce Aluminum Oxide Chemical Equation Aluminum Oxide Yields Aluminum And Oxygen Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Al + o2 → al2o3. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Since metallic aluminum is very reactive with atmospheric.. Aluminum Oxide Yields Aluminum And Oxygen.
From www.coursehero.com
[Solved] What is the theoretical yield of aluminum oxide if 2.40 mol of Aluminum Oxide Yields Aluminum And Oxygen Aluminium burns in oxygen to form al2o3. Aluminum oxide is formed when aluminum reacts with oxygen. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Achieve a common 6 oxygen atoms on either side of the. Al + o2 → al2o3. Instead, it turns out to be much easier to simply force. Aluminum Oxide Yields Aluminum And Oxygen.
From www.youtube.com
Give formation of Aluminium oxide Formation of aluminium oxide by Aluminum Oxide Yields Aluminum And Oxygen Al + o2 → al2o3. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. In both cases, the metal acquires a positive charge by transferring. Since metallic aluminum is very reactive with atmospheric. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum. Aluminum Oxide Yields Aluminum And Oxygen.
From www.w3schools.blog
Aluminium Extraction W3schools Aluminum Oxide Yields Aluminum And Oxygen Aluminum oxide is formed when aluminum reacts with oxygen. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential.. Aluminum Oxide Yields Aluminum And Oxygen.
From www.chegg.com
Solved Part A What is the theoretical yield of aluminum Aluminum Oxide Yields Aluminum And Oxygen Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? In both cases, the metal acquires a positive charge by transferring. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Your solution’s ready to go! Aluminium burns in oxygen to form al2o3. Al + o2 → al2o3. This is. Aluminum Oxide Yields Aluminum And Oxygen.
From solvedlib.com
Solid aluminum and gaseous oxygen react in a combinat… SolvedLib Aluminum Oxide Yields Aluminum And Oxygen In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. This is the unbalanced equation described. Al + o2 → al2o3. Since metallic aluminum is very reactive with atmospheric. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface.. Aluminum Oxide Yields Aluminum And Oxygen.
From www.chegg.com
Solved Aluminum reacts with oxygen to produce aluminum oxide Aluminum Oxide Yields Aluminum And Oxygen Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Aluminium burns in oxygen to form al2o3. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum. Aluminum Oxide Yields Aluminum And Oxygen.
From www.bartleby.com
Answered Solid aluminum and gaseous oxygen react… bartleby Aluminum Oxide Yields Aluminum And Oxygen Aluminum oxide is formed when aluminum reacts with oxygen. Aluminium burns in oxygen to form al2o3. Since metallic aluminum is very reactive with atmospheric. This is the unbalanced equation described. Your solution’s ready to go! Aluminum oxide is responsible for resistance of metallic aluminum to weathering. In both cases, the metal acquires a positive charge by transferring. Al + o2. Aluminum Oxide Yields Aluminum And Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! Aluminium burns in oxygen to form al2o3. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Achieve a common 6 oxygen atoms on either side of the. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide. Aluminum Oxide Yields Aluminum And Oxygen.
From www.numerade.com
SOLVED Solid aluminum and gaseous oxygen react in a combination to Aluminum Oxide Yields Aluminum And Oxygen In both cases, the metal acquires a positive charge by transferring. Al + o2 → al2o3. This is the unbalanced equation described. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Aluminum and oxygen react. Aluminum Oxide Yields Aluminum And Oxygen.
From www.youtube.com
008 Aluminum + Oxygen = Aluminum Oxide YouTube Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! Since metallic aluminum is very reactive with atmospheric. Aluminum oxide is formed when aluminum reacts with oxygen. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Instead, it turns out to be much easier to simply. Aluminum Oxide Yields Aluminum And Oxygen.
From www.numerade.com
Aluminum reacts with oxygen to yield aluminum oxide. If 5.0 g of Al Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Aluminum oxide is formed when aluminum reacts with oxygen. When exposed to air, aluminum metal. Aluminum Oxide Yields Aluminum And Oxygen.
From www.numerade.com
SOLVED Write the balanced equation for the formation of aluminum oxide Aluminum Oxide Yields Aluminum And Oxygen In both cases, the metal acquires a positive charge by transferring. Aluminium burns in oxygen to form al2o3. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Al + o2 → al2o3. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? This is the unbalanced equation described. Aluminum. Aluminum Oxide Yields Aluminum And Oxygen.
From www.chegg.com
Solved Solid aluminum and gaseous oxygen react in a Aluminum Oxide Yields Aluminum And Oxygen Achieve a common 6 oxygen atoms on either side of the. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. In both cases, the metal acquires a positive charge by transferring. Instead, it turns out to be much easier. Aluminum Oxide Yields Aluminum And Oxygen.
From www.youtube.com
Extraction of Aluminium (Electrolysis of Aluminium Oxide) GCSE Aluminum Oxide Yields Aluminum And Oxygen Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Achieve a common 6 oxygen atoms on either side of the. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Al + o2 → al2o3. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. Since metallic aluminum. Aluminum Oxide Yields Aluminum And Oxygen.
From www.pw.live
Aluminium Oxide Formula, Structure, Properties, Uses Aluminum Oxide Yields Aluminum And Oxygen Aluminum oxide is formed when aluminum reacts with oxygen. Achieve a common 6 oxygen atoms on either side of the. Your solution’s ready to go! This is the unbalanced equation described. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. When exposed to air, aluminum metal. Aluminum Oxide Yields Aluminum And Oxygen.
From www.numerade.com
SOLVED Consider the reaction of aluminium and oxygen. 1. What is the Aluminum Oxide Yields Aluminum And Oxygen Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Aluminium burns in oxygen to form al2o3. Al + o2 → al2o3. Aluminum oxide is formed when aluminum reacts with oxygen. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Achieve a common 6 oxygen atoms on either side. Aluminum Oxide Yields Aluminum And Oxygen.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID4539778 Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! Aluminium burns in oxygen to form al2o3. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. In both cases, the metal acquires a positive charge by transferring. Aluminum oxide is formed when aluminum reacts. Aluminum Oxide Yields Aluminum And Oxygen.
From www.slideserve.com
PPT Word equations to balanced chemical equations drill Each of the Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Achieve a common 6 oxygen atoms on either side of the. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Aluminium burns in oxygen to form al2o3. When. Aluminum Oxide Yields Aluminum And Oxygen.
From www.coursehero.com
[Solved] Solid aluminum (Al)and oxygen (O2) gas react to form solid Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! Achieve a common 6 oxygen atoms on either side of the. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Aluminum oxide is responsible for resistance of metallic aluminum to weathering. In both cases, the metal acquires a positive charge by transferring. Instead, it turns out to. Aluminum Oxide Yields Aluminum And Oxygen.
From www.numerade.com
SOLVED Solid aluminum and gaseous oxygen react in a synthesis reaction Aluminum Oxide Yields Aluminum And Oxygen Your solution’s ready to go! Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. In both cases, the metal acquires a positive charge by transferring. Aluminium burns in oxygen to form al2o3. Instead, it turns. Aluminum Oxide Yields Aluminum And Oxygen.
From www.numerade.com
SOLVED Solid aluminum and gaseous oxygen react in a combination Aluminum Oxide Yields Aluminum And Oxygen Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Since metallic aluminum is very reactive with atmospheric. Your solution’s ready to go! Aluminum oxide is formed when aluminum reacts with oxygen. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Instead, it turns out to be much easier to simply. Aluminum Oxide Yields Aluminum And Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas Aluminum Oxide Yields Aluminum And Oxygen In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Al + o2 → al2o3. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. Since metallic aluminum is very reactive with atmospheric. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3?. Aluminum Oxide Yields Aluminum And Oxygen.
From www.slideserve.com
PPT Unit 8 Chemical Reactions PowerPoint Presentation, free download Aluminum Oxide Yields Aluminum And Oxygen In both cases, the metal acquires a positive charge by transferring. Aluminum oxide is formed when aluminum reacts with oxygen. This is the unbalanced equation described. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Al + o2 → al2o3. Aluminium burns in oxygen to form al2o3. Since metallic aluminum is very. Aluminum Oxide Yields Aluminum And Oxygen.
From www.bartleby.com
Answered Metallic aluminum is easily oxidized by… bartleby Aluminum Oxide Yields Aluminum And Oxygen Aluminum oxide is formed when aluminum reacts with oxygen. In both cases, the metal acquires a positive charge by transferring. This is the unbalanced equation described. Al + o2 → al2o3. Aluminium burns in oxygen to form al2o3. Aluminum and oxygen react to form aluminum oxide by the equation.what is the percent yield of ai2o3? Instead, it turns out to. Aluminum Oxide Yields Aluminum And Oxygen.
From www.slideserve.com
PPT What is the difference between a chemical reaction and physical Aluminum Oxide Yields Aluminum And Oxygen Achieve a common 6 oxygen atoms on either side of the. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. Your solution’s ready to go! Since metallic aluminum is very reactive with atmospheric. Aluminum oxide is formed when aluminum reacts with oxygen. Aluminum oxide is responsible. Aluminum Oxide Yields Aluminum And Oxygen.
From www.numerade.com
SOLVED Aluminum metal reacts with oxygen gas to produce aluminum oxide Aluminum Oxide Yields Aluminum And Oxygen Aluminium burns in oxygen to form al2o3. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. In terms thu7o2ng, aluminum reacts with oxygen to form thin al2o3 layer sustainability. Aluminum oxide is formed when aluminum reacts with oxygen. Aluminum oxide is responsible for resistance of metallic aluminum to weathering. Instead, it turns. Aluminum Oxide Yields Aluminum And Oxygen.
From bernard-blogrose.blogspot.com
Aluminum Reacts With Oxygen to Produce Aluminum Oxide Chemical Equation Aluminum Oxide Yields Aluminum And Oxygen In both cases, the metal acquires a positive charge by transferring. Instead, it turns out to be much easier to simply force oxygen to give its electrons back to aluminum using an electric potential. This is the unbalanced equation described. When exposed to air, aluminum metal develops a continuous, transparent layer of aluminum oxide on its surface. Aluminum oxide is. Aluminum Oxide Yields Aluminum And Oxygen.