What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun . This facilitates the formation of crystals by speeding the. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. The solution is cooled in open air first, and then cooled in an ice bath. Slow cooling often leads to purer crystals. recrystallisation may be done in a multitude of ways, depending on your particular situation; it works because: the purpose of placing something in an ice bath is to cool it. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. In most cases, this will be done after the reaction or after. if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the.
from rimbasweat.com.au
1) different substances have different solubilities in the same solvent, and 2) only molecules of. This facilitates the formation of crystals by speeding the. The solution is cooled in open air first, and then cooled in an ice bath. Slow cooling often leads to purer crystals. In most cases, this will be done after the reaction or after. it works because: after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. recrystallisation may be done in a multitude of ways, depending on your particular situation; the purpose of placing something in an ice bath is to cool it.
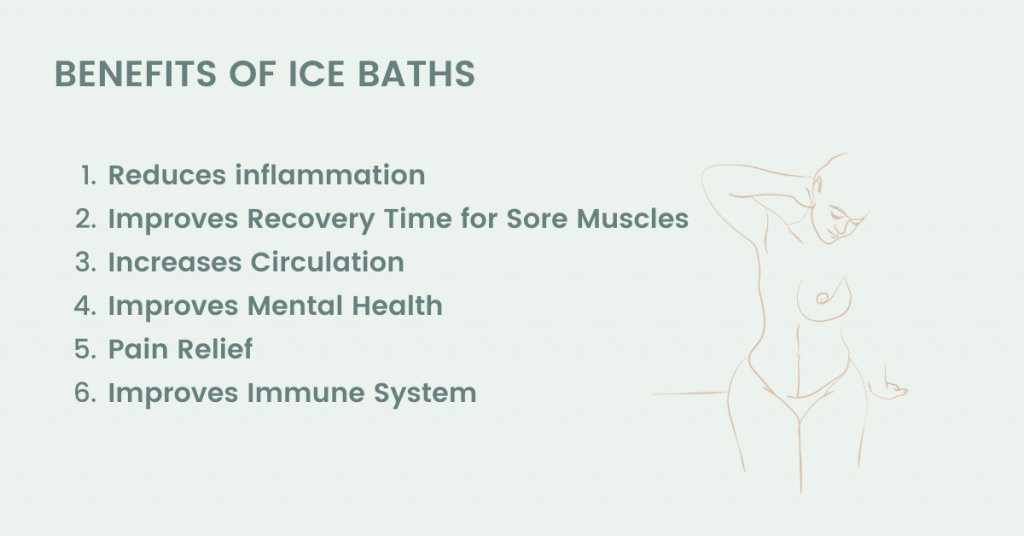
What Are the Benefits of Ice Baths? Rimba Sweat
What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. the purpose of placing something in an ice bath is to cool it. In most cases, this will be done after the reaction or after. Slow cooling often leads to purer crystals. Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. The solution is cooled in open air first, and then cooled in an ice bath. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. recrystallisation may be done in a multitude of ways, depending on your particular situation; This facilitates the formation of crystals by speeding the. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. it works because:
From www.pinterest.com
The Cold Truth About Taking an Ice Bath After Exercise Ice bath What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun 1) different substances have different solubilities in the same solvent, and 2) only molecules of. the purpose of placing something in an ice bath is to cool it. recrystallisation may be done in a multitude of ways, depending on your particular situation; This facilitates the formation of crystals by speeding the. if no crystals form even after. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From blog.joinfightcamp.com
5 Ice Bath Benefits For Recovery FightCamp What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun In most cases, this will be done after the reaction or after. recrystallisation may be done in a multitude of ways, depending on your particular situation; if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. after the solution has come to. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From marathonhandbook.com
6 Ice Bath Benefits + What Is The Optimal Time To Stay In? What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun The solution is cooled in open air first, and then cooled in an ice bath. Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. the purpose of placing. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From maxtorc.com
How to Maximize the Benefits of an Ice Bath What to Do Before and What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun the purpose of placing something in an ice bath is to cool it. it works because: recrystallisation may be done in a multitude of ways, depending on your particular situation; Slow cooling often leads to purer crystals. This facilitates the formation of crystals by speeding the. 1) different substances have different solubilities in the same solvent, and. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.samasati.com
Benefits of an Ice Bath (12 Listed) Is It Good For You? What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun 1) different substances have different solubilities in the same solvent, and 2) only molecules of. the purpose of placing something in an ice bath is to cool it. recrystallisation may be done in a multitude of ways, depending on your particular situation; Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From collegedunia.com
Crystallization Definition, Process, Types & Examples What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun it works because: This facilitates the formation of crystals by speeding the. Slow cooling often leads to purer crystals. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. The solution is. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.vikasati.com.au
5 Ice Bath Benefits The Cold Truth Revealed What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. it works because: This facilitates the formation of crystals by speeding the. Slow cooling often leads to purer crystals. The solution is cooled in open air first, and then cooled in an ice bath. In most cases,. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.ninjawarriorx.com
Ice Bath The Benefits and How to Make It Right (Guide) What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun Slow cooling often leads to purer crystals. it works because: 1) different substances have different solubilities in the same solvent, and 2) only molecules of. recrystallisation may be done in a multitude of ways, depending on your particular situation; if no crystals form even after the solution has been cooled in an ice bath, take a fire. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From pluslifehealth.ae
Benefits of Ice Baths and Cold Plunges What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun recrystallisation may be done in a multitude of ways, depending on your particular situation; the purpose of placing something in an ice bath is to cool it. if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. In most cases, this will. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From marathonhandbook.com
6 Ice Bath Benefits + What Is The Optimal Time To Stay In? What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun 1) different substances have different solubilities in the same solvent, and 2) only molecules of. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. recrystallisation may be done in a multitude of ways, depending on your particular situation; the purpose of placing something in an. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From ar.inspiredpencil.com
Ice Bath Chemistry What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun In most cases, this will be done after the reaction or after. if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. The solution is cooled in open air first, and then cooled in an ice bath. it works because: after the. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.youtube.com
Creating a Proper Ice Bath.mp4 YouTube What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun the purpose of placing something in an ice bath is to cool it. it works because: The solution is cooled in open air first, and then cooled in an ice bath. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. recrystallisation may be done in a multitude of ways, depending on. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From recovatech.com
A Quick Guide to Finding The Ideal Ice Bath Temperature Recovatech What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun the purpose of placing something in an ice bath is to cool it. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. it works because: The solution is cooled in. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.youtube.com
Ice Bath For Faster Recovery Benefits Of Taking An Ice Bath YouTube What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun This facilitates the formation of crystals by speeding the. Slow cooling often leads to purer crystals. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From craftsy.life
20 DIY Ice Bath Ideas How To Make An Ice Bath At Home Craftsy What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. recrystallisation may be done in a multitude of ways, depending on your particular situation; Slow cooling often leads to purer crystals. 1) different substances have different solubilities in the same solvent, and 2). What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.aakash.ac.in
Crystallization Process, Precautions, Advantages & Examples What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun The solution is cooled in open air first, and then cooled in an ice bath. recrystallisation may be done in a multitude of ways, depending on your particular situation; if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. This facilitates the formation. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.geeksforgeeks.org
Crystallization Definition, Process, Types, and Examples What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun recrystallisation may be done in a multitude of ways, depending on your particular situation; Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. This facilitates the formation of crystals by speeding the. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. Slow cooling often. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.artofit.org
33 benefits of ice baths Artofit What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun Slow cooling often leads to purer crystals. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. it works because: the purpose of placing something in an ice bath is to cool it. Develop the skill to perform a recrystallization effectively, including the use of minimal. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.slideserve.com
PPT Recrystallization Lab PowerPoint Presentation ID830188 What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun Slow cooling often leads to purer crystals. In most cases, this will be done after the reaction or after. The solution is cooled in open air first, and then cooled in an ice bath. recrystallisation may be done in a multitude of ways, depending on your particular situation; after the solution has come to room temperature, it is. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.orangefit.eu
6 Proven Benefits of Ice Baths or Cold Showers (+ Tips) What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun the purpose of placing something in an ice bath is to cool it. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. This facilitates the formation of crystals by speeding the. The solution is cooled in open air first, and then cooled in an ice bath.. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From medicaldarpan.com
Excellent health benefits of Taking an ice bath! Medical Darpan What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun 1) different substances have different solubilities in the same solvent, and 2) only molecules of. The solution is cooled in open air first, and then cooled in an ice bath. recrystallisation may be done in a multitude of ways, depending on your particular situation; This facilitates the formation of crystals by speeding the. after the solution has come. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From lulelaboratory.blogspot.com
Lu Le Laboratory Crystallization Techniques in Organic Chemistry What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun This facilitates the formation of crystals by speeding the. Slow cooling often leads to purer crystals. In most cases, this will be done after the reaction or after. recrystallisation may be done in a multitude of ways, depending on your particular situation; the purpose of placing something in an ice bath is to cool it. if no. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.dnaindia.com
8 Benefits of ice bath What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun This facilitates the formation of crystals by speeding the. Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. it works because: the purpose of placing something in an ice bath is to cool it. recrystallisation may be done in a multitude of ways, depending on your particular situation;. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.benchmarkmonitor.com
3 Interesting Benefits of Ice Bath That You Must Know Today Benchmark What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. Slow. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From ultimatenutrition.com
Top 8 Benefits of an Ice Bath Ultimate Nutrition What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun This facilitates the formation of crystals by speeding the. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. The solution is cooled in open air first, and then cooled in an ice. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From dxoehvsap.blob.core.windows.net
How To Get Enough Ice For An Ice Bath at Dennis Appel blog What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun In most cases, this will be done after the reaction or after. The solution is cooled in open air first, and then cooled in an ice bath. Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. 1) different substances have different solubilities in the same solvent, and 2) only molecules of.. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From blog.torokhtiy.com
What To Do After An Ice Bath To Maximize Its Benefits What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. The solution is cooled in open air first, and then cooled in an ice bath. Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. In most cases, this will. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From rimbasweat.com.au
What Are the Benefits of Ice Baths? Rimba Sweat What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun the purpose of placing something in an ice bath is to cool it. In most cases, this will be done after the reaction or after. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. The. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.tffn.net
The Benefits of Ice Baths Why You Should Consider Adding an Ice Bath What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun 1) different substances have different solubilities in the same solvent, and 2) only molecules of. the purpose of placing something in an ice bath is to cool it. This facilitates the formation of crystals by speeding the. Slow cooling often leads to purer crystals. recrystallisation may be done in a multitude of ways, depending on your particular situation;. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From ignorelimits.com
Ice Bath Benefits 7 Reasons Why You Need To Incorporate Ice Baths Into What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun Slow cooling often leads to purer crystals. if no crystals form even after the solution has been cooled in an ice bath, take a fire polished stirring rod and etch (scratch) the. the purpose of placing something in an ice bath is to cool it. recrystallisation may be done in a multitude of ways, depending on your. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.cetcryospas.com
Ice bath benefits Why to use ice baths after sports What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if. the purpose of placing something in an ice bath is to cool it. recrystallisation may be done in a multitude of ways, depending on your particular situation; 1) different substances have different solubilities in the same solvent, and 2) only. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.homebrewfinds.com
Critical Steps to Creating a Proper Ice Bath Homebrew Finds What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. The solution is cooled in open air first, and then cooled in an ice bath. it works because: In most cases, this will be done after the reaction or after. recrystallisation may be done in a. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.chefstemp.com
How to Create a Properly Made Ice Bath? ChefsTemp What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun the purpose of placing something in an ice bath is to cool it. In most cases, this will be done after the reaction or after. recrystallisation may be done in a multitude of ways, depending on your particular situation; if no crystals form even after the solution has been cooled in an ice bath, take a fire. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.orbitfitness.com.au
Ice Bath Benefits The Real Deal or Just Hype? Orbit Fitness What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun In most cases, this will be done after the reaction or after. 1) different substances have different solubilities in the same solvent, and 2) only molecules of. This facilitates the formation of crystals by speeding the. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. if. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.
From www.valuefood.info
Benefits Of Ice Bath Facts And Researched DataValue Food What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun Slow cooling often leads to purer crystals. The solution is cooled in open air first, and then cooled in an ice bath. it works because: This facilitates the formation of crystals by speeding the. after the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. Develop the skill. What Is The Purpose Of Utilizing An Ice Bath After Crystallization Has Begun.