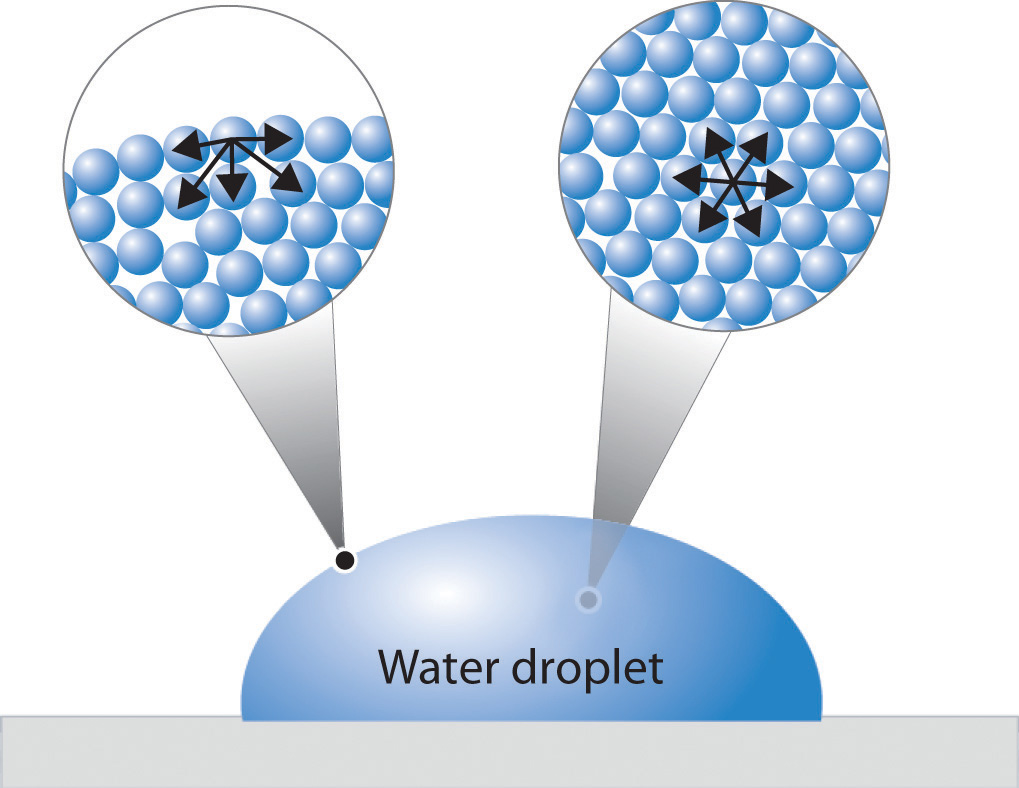
How Does Surface Tension Of A Liquid Vary With Temperature . It becomes zero at the critical. As might be expected, the coefficient of surface tension decreases with increasing temperature. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Define viscosity, surface tension, and capillary rise; The answer lies in a property called surface tension, which depends on intermolecular forces. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Variation of surface tension with temperature. In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1. Describe the roles of intermolecular attractive forces in each of these.
from chem.libretexts.org
If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. Describe the roles of intermolecular attractive forces in each of these. Define viscosity, surface tension, and capillary rise; Variation of surface tension with temperature. It becomes zero at the critical. As might be expected, the coefficient of surface tension decreases with increasing temperature. The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1. The answer lies in a property called surface tension, which depends on intermolecular forces.
12.2 Some Properties of Liquids Chemistry LibreTexts
How Does Surface Tension Of A Liquid Vary With Temperature It becomes zero at the critical. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Describe the roles of intermolecular attractive forces in each of these. Variation of surface tension with temperature. The answer lies in a property called surface tension, which depends on intermolecular forces. Define viscosity, surface tension, and capillary rise; In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. As might be expected, the coefficient of surface tension decreases with increasing temperature. It becomes zero at the critical. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1.
From aznswerzonelinclarifying.z21.web.core.windows.net
How To Explain Surface Tension How Does Surface Tension Of A Liquid Vary With Temperature The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. The answer lies in a property called surface tension, which depends on intermolecular forces. It becomes zero at the critical. Describe the roles. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.researchgate.net
Relationship between temperature and surface tension of liquid water How Does Surface Tension Of A Liquid Vary With Temperature Define viscosity, surface tension, and capillary rise; The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1. In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. The answer lies in a property called surface tension, which depends on. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry How Does Surface Tension Of A Liquid Vary With Temperature In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? As might be expected, the coefficient of surface tension decreases with. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.quora.com
What is the effect of temperature on the surface tension, viscosity How Does Surface Tension Of A Liquid Vary With Temperature Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Describe the roles of intermolecular attractive forces in each of these. Variation of surface tension with temperature. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. The answer lies in. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint How Does Surface Tension Of A Liquid Vary With Temperature If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Describe the roles of intermolecular attractive forces in each of these. Define viscosity, surface tension, and capillary rise; The answer lies in a property called surface tension, which depends. How Does Surface Tension Of A Liquid Vary With Temperature.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Does Surface Tension Of A Liquid Vary With Temperature Describe the roles of intermolecular attractive forces in each of these. Variation of surface tension with temperature. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Define viscosity, surface tension,. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.toppr.com
How does surface tension of a liquid vary with temperature?or Define v How Does Surface Tension Of A Liquid Vary With Temperature Variation of surface tension with temperature. As might be expected, the coefficient of surface tension decreases with increasing temperature. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.quora.com
Does temperature increase surface tension? Quora How Does Surface Tension Of A Liquid Vary With Temperature As might be expected, the coefficient of surface tension decreases with increasing temperature. Describe the roles of intermolecular attractive forces in each of these. Define viscosity, surface tension, and capillary rise; It becomes zero at the critical. The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1.. How Does Surface Tension Of A Liquid Vary With Temperature.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Does Surface Tension Of A Liquid Vary With Temperature The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Variation of surface tension with temperature. The answer lies in a property called surface tension, which depends on intermolecular forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Define. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Of A Liquid Vary With Temperature Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It becomes zero at the critical. The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1. As might be expected, the coefficient of surface tension decreases with. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.thoughtco.com
Liquid Definition and Examples (Chemistry) How Does Surface Tension Of A Liquid Vary With Temperature Variation of surface tension with temperature. In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. Describe the roles of intermolecular attractive forces in each of these. As might be expected, the coefficient of surface tension decreases with increasing temperature. The work done on the liquid is 2 a γ 1 ∆. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Of A Liquid Vary With Temperature The answer lies in a property called surface tension, which depends on intermolecular forces. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Define viscosity, surface tension, and capillary rise; As might be expected, the coefficient of surface tension decreases with increasing temperature. It becomes zero at the critical. The. How Does Surface Tension Of A Liquid Vary With Temperature.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru How Does Surface Tension Of A Liquid Vary With Temperature It becomes zero at the critical. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Variation of surface tension with temperature. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Define viscosity, surface tension, and capillary rise; The answer. How Does Surface Tension Of A Liquid Vary With Temperature.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Of A Liquid Vary With Temperature In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of.. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.youtube.com
What is Effect Of Temperature on surface tension Properties of How Does Surface Tension Of A Liquid Vary With Temperature Variation of surface tension with temperature. The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1. Describe the roles of intermolecular attractive forces in each of these. The answer lies in a property called surface tension, which depends on intermolecular forces. Define viscosity, surface tension, and capillary. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.meritnation.com
How does the surface tension of liquid vary with temperature Physics How Does Surface Tension Of A Liquid Vary With Temperature Describe the roles of intermolecular attractive forces in each of these. It becomes zero at the critical. In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Variation of surface tension with temperature.. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Does Surface Tension Of A Liquid Vary With Temperature The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. The answer lies in a property called surface tension, which depends on intermolecular forces. Define viscosity, surface tension, and capillary rise; If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Of A Liquid Vary With Temperature If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Define viscosity, surface tension, and capillary rise; Variation of surface tension with temperature. As might be expected, the coefficient of surface tension decreases with increasing temperature. Surface tension is. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.youtube.com
What is Surface Tension Liquid State Liquid Dynamics Surface How Does Surface Tension Of A Liquid Vary With Temperature The answer lies in a property called surface tension, which depends on intermolecular forces. Variation of surface tension with temperature. In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. As might be expected, the coefficient of surface tension decreases with increasing temperature. The way a fluid behaves—how it flows, how it. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co How Does Surface Tension Of A Liquid Vary With Temperature Define viscosity, surface tension, and capillary rise; The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. It becomes zero at the critical. Variation of surface tension with temperature. Describe the roles of intermolecular attractive forces in each of these. As might be expected, the coefficient of surface tension decreases with. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary How Does Surface Tension Of A Liquid Vary With Temperature Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Define viscosity, surface tension, and capillary rise; It becomes. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 How Does Surface Tension Of A Liquid Vary With Temperature The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Define viscosity, surface tension, and capillary rise; The answer lies in a property called surface tension, which depends on intermolecular forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free How Does Surface Tension Of A Liquid Vary With Temperature The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Describe the roles of intermolecular attractive forces in each of these. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Of A Liquid Vary With Temperature Variation of surface tension with temperature. In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. Describe the roles of intermolecular attractive forces in each of these. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. As might be expected, the coefficient. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free How Does Surface Tension Of A Liquid Vary With Temperature Define viscosity, surface tension, and capillary rise; In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. Describe the roles of intermolecular attractive forces in each of these. As might be expected, the coefficient of surface tension decreases with increasing temperature. If liquids tend to adopt the shapes of their containers, then. How Does Surface Tension Of A Liquid Vary With Temperature.
From chem.libretexts.org
12.2 Some Properties of Liquids Chemistry LibreTexts How Does Surface Tension Of A Liquid Vary With Temperature The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1. The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. Variation of surface tension with temperature. In order that the process should be isothermal, the liquid has to. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID How Does Surface Tension Of A Liquid Vary With Temperature The work done on the liquid is 2 a γ 1 ∆ x, where γ 1 is the surface tension at temperature t1. As might be expected, the coefficient of surface tension decreases with increasing temperature. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Define viscosity, surface tension,. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.youtube.com
Determination of surface tension of Liquid by using stalagmometer How Does Surface Tension Of A Liquid Vary With Temperature The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. As might be expected, the coefficient of surface tension decreases with increasing temperature. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Surface Tension Of A Liquid Vary With Temperature Describe the roles of intermolecular attractive forces in each of these. The answer lies in a property called surface tension, which depends on intermolecular forces. It becomes zero at the critical. As might be expected, the coefficient of surface tension decreases with increasing temperature. The work done on the liquid is 2 a γ 1 ∆ x, where γ 1. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.logiota.com
Surface Tension States of Matter Physical Chemistry Chemistry How Does Surface Tension Of A Liquid Vary With Temperature In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Describe the roles of intermolecular attractive forces in each of these.. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Of A Liquid Vary With Temperature In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. As might be expected, the coefficient of surface tension decreases with increasing temperature. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The work done on the liquid is 2 a. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How Does Surface Tension Of A Liquid Vary With Temperature If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a thin, continuous film? Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The work done on the liquid is 2 a. How Does Surface Tension Of A Liquid Vary With Temperature.
From readchemistry.com
Determination of Surface Tension Read Chemistry How Does Surface Tension Of A Liquid Vary With Temperature Define viscosity, surface tension, and capillary rise; In order that the process should be isothermal, the liquid has to absorb an amount of heat q1. Variation of surface tension with temperature. The answer lies in a property called surface tension, which depends on intermolecular forces. Surface tension is the energy, or work, required to increase the surface area of a. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.researchgate.net
Relationship between temperature and viscosity of liquid water How Does Surface Tension Of A Liquid Vary With Temperature Describe the roles of intermolecular attractive forces in each of these. It becomes zero at the critical. Define viscosity, surface tension, and capillary rise; As might be expected, the coefficient of surface tension decreases with increasing temperature. Variation of surface tension with temperature. If liquids tend to adopt the shapes of their containers, then why do small amounts of water. How Does Surface Tension Of A Liquid Vary With Temperature.
From www.youtube.com
Surface Tension on Liquid Jet formula derivation with 3d animation How Does Surface Tension Of A Liquid Vary With Temperature Define viscosity, surface tension, and capillary rise; The way a fluid behaves—how it flows, how it rests, and how it nucleates—is influenced by the magnitude of. It becomes zero at the critical. If liquids tend to adopt the shapes of their containers, then why do small amounts of water on a freshly waxed car form raised droplets instead of a. How Does Surface Tension Of A Liquid Vary With Temperature.