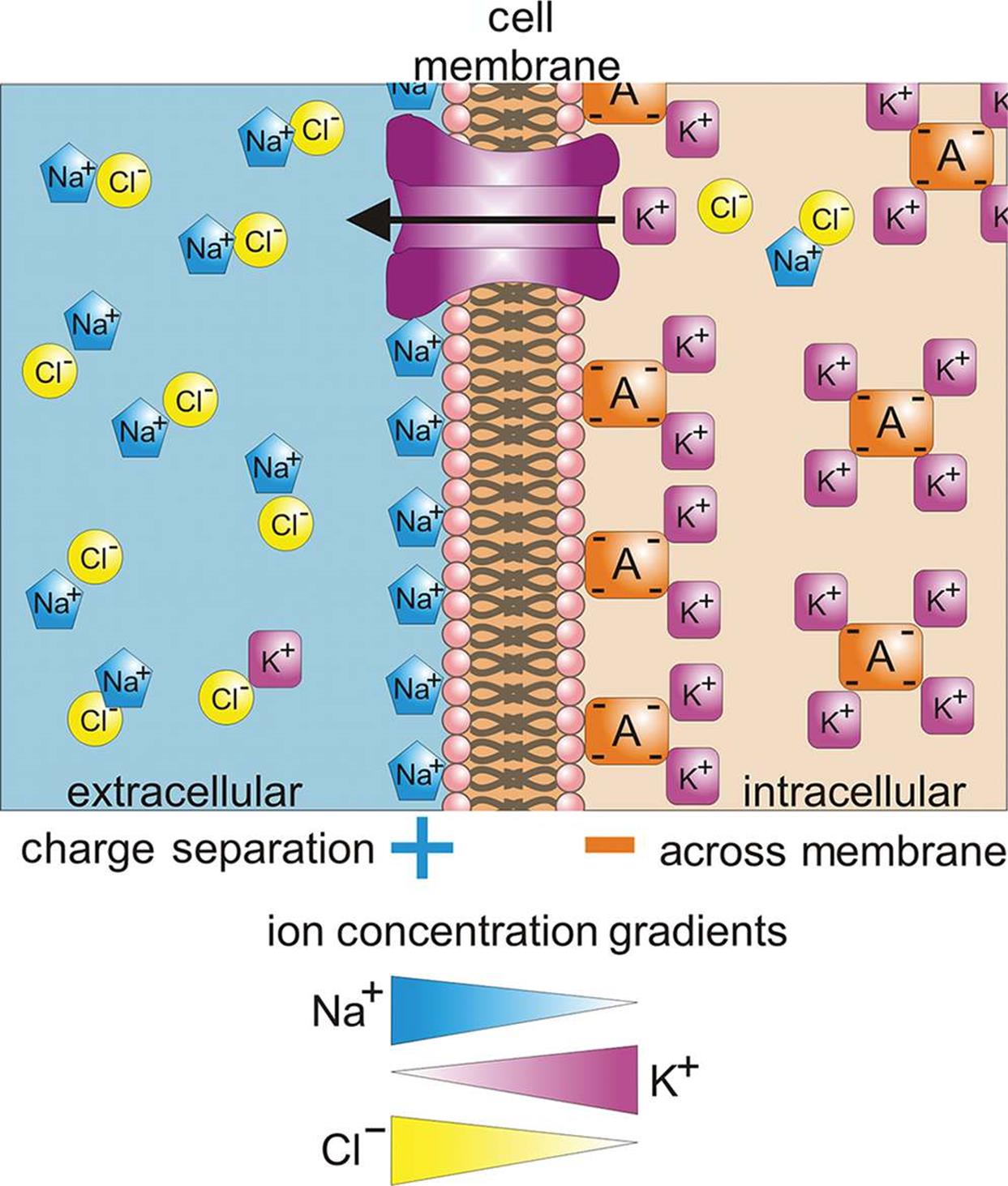
Why Is The Electrochemical Gradient Important For Cellular Function . The electrochemical gradients are vital elements in numerous cellular processes,. This energy is harvested from. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. Role of electrochemical gradients in cellular functions. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. Adenosine triphosphate, or atp, is known as the primary. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The importance of the electrochemical gradient is highlighted by the following points: To move substances against a concentration or electrochemical gradient, the cell must use energy.
from schoolbag.info
The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The electrochemical gradients are vital elements in numerous cellular processes,. Role of electrochemical gradients in cellular functions. The importance of the electrochemical gradient is highlighted by the following points: To move substances against a concentration or electrochemical gradient, the cell must use energy. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. This energy is harvested from. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. Adenosine triphosphate, or atp, is known as the primary.
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell
Why Is The Electrochemical Gradient Important For Cellular Function The importance of the electrochemical gradient is highlighted by the following points: The importance of the electrochemical gradient is highlighted by the following points: The electrochemical gradients are vital elements in numerous cellular processes,. This energy is harvested from. Role of electrochemical gradients in cellular functions. Adenosine triphosphate, or atp, is known as the primary. To move substances against a concentration or electrochemical gradient, the cell must use energy. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 Why Is The Electrochemical Gradient Important For Cellular Function Adenosine triphosphate, or atp, is known as the primary. The electrochemical gradients are vital elements in numerous cellular processes,. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. This energy is harvested from. Role of electrochemical gradients in cellular functions.. Why Is The Electrochemical Gradient Important For Cellular Function.
From teachmephysiology.com
Resting Membrane Potential Nernst Generation TeachMePhysiology Why Is The Electrochemical Gradient Important For Cellular Function The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. The electrochemical gradients are vital elements in numerous cellular processes,. This energy is harvested from. The electrochemical gradient determines the direction that ions will flow through an open ion channel and. Why Is The Electrochemical Gradient Important For Cellular Function.
From alg.manifoldapp.org
“Chapter 8 The Electrochemical Gradient” in “Fundamentals of Cell Why Is The Electrochemical Gradient Important For Cellular Function The electrochemical gradients are vital elements in numerous cellular processes,. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. This energy is harvested from. Adenosine triphosphate, or atp, is known as the primary. To move substances against a concentration or. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.macmillanhighered.com
Figure 25.10 Why Is The Electrochemical Gradient Important For Cellular Function The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. To move substances against a concentration or electrochemical gradient, the cell must use energy. The electrochemical gradients are vital elements in numerous cellular processes,. Adenosine triphosphate, or atp, is known as the primary. The precise mechanisms by. Why Is The Electrochemical Gradient Important For Cellular Function.
From rwu.pressbooks.pub
Chapter 8. Membrane Transport Introduction to Molecular and Cell Biology Why Is The Electrochemical Gradient Important For Cellular Function The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. Adenosine triphosphate, or atp, is known as the primary. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells.. Why Is The Electrochemical Gradient Important For Cellular Function.
From alg.manifoldapp.org
“Chapter 8 The Electrochemical Gradient” in “Fundamentals of Cell Why Is The Electrochemical Gradient Important For Cellular Function Adenosine triphosphate, or atp, is known as the primary. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The importance of the electrochemical gradient. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.youtube.com
3.2d Electrochemical Gradient across Cell Membrane (हिंदी में Why Is The Electrochemical Gradient Important For Cellular Function This energy is harvested from. Adenosine triphosphate, or atp, is known as the primary. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. To move substances against a concentration or electrochemical gradient, the cell must use energy. Role of electrochemical. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.numerade.com
SOLVED The direction of the electrochemical gradient for Na is the Why Is The Electrochemical Gradient Important For Cellular Function The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. This energy is harvested from. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. Adenosine triphosphate, or. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.mathwizurd.com
Electron Transport Chain — Mathwizurd Why Is The Electrochemical Gradient Important For Cellular Function The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The electrochemical gradients are vital elements in numerous cellular processes,. Adenosine triphosphate, or atp, is known as the primary. The importance of the electrochemical gradient is highlighted by the following points: The precise mechanisms by which a proton. Why Is The Electrochemical Gradient Important For Cellular Function.
From bio.libretexts.org
2.2.4 Active Transport Biology LibreTexts Why Is The Electrochemical Gradient Important For Cellular Function The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. Adenosine triphosphate, or atp, is known as the primary. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types. Why Is The Electrochemical Gradient Important For Cellular Function.
From quizlet.com
Electrochemical Gradient Diagram Quizlet Why Is The Electrochemical Gradient Important For Cellular Function Adenosine triphosphate, or atp, is known as the primary. The electrochemical gradients are vital elements in numerous cellular processes,. Role of electrochemical gradients in cellular functions. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The importance of the electrochemical gradient is highlighted by the following points:. Why Is The Electrochemical Gradient Important For Cellular Function.
From redroverpetcare.com
Active Transport Definition, Types, Process, Functions, Examples (2023) Why Is The Electrochemical Gradient Important For Cellular Function To move substances against a concentration or electrochemical gradient, the cell must use energy. Adenosine triphosphate, or atp, is known as the primary. This energy is harvested from. The electrochemical gradients are vital elements in numerous cellular processes,. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.slideserve.com
PPT Animal Cell PowerPoint Presentation, free download ID9481857 Why Is The Electrochemical Gradient Important For Cellular Function Role of electrochemical gradients in cellular functions. To move substances against a concentration or electrochemical gradient, the cell must use energy. The electrochemical gradients are vital elements in numerous cellular processes,. This energy is harvested from. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.drawittoknowit.com
General Biology Glossary Electrochemical Gradient Draw It to Know It Why Is The Electrochemical Gradient Important For Cellular Function The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The importance of the electrochemical gradient is highlighted by the following points: The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. Adenosine triphosphate, or. Why Is The Electrochemical Gradient Important For Cellular Function.
From 2012books.lardbucket.org
Describing Electrochemical Cells Why Is The Electrochemical Gradient Important For Cellular Function Adenosine triphosphate, or atp, is known as the primary. The importance of the electrochemical gradient is highlighted by the following points: This energy is harvested from. Role of electrochemical gradients in cellular functions. The electrochemical gradients are vital elements in numerous cellular processes,. To move substances against a concentration or electrochemical gradient, the cell must use energy. The combined gradient. Why Is The Electrochemical Gradient Important For Cellular Function.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Why Is The Electrochemical Gradient Important For Cellular Function The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. The electrochemical gradients are vital elements in numerous cellular processes,. This energy is harvested from. Role of electrochemical gradients in cellular functions. The combined gradient that affects an ion is called. Why Is The Electrochemical Gradient Important For Cellular Function.
From neurotext.library.stonybrook.edu
Cellular Neurophysiology Why Is The Electrochemical Gradient Important For Cellular Function Adenosine triphosphate, or atp, is known as the primary. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy is harvested from. The electrochemical gradients. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.pearson.com
Electrochemical Gradient Channels for Pearson+ Why Is The Electrochemical Gradient Important For Cellular Function This energy is harvested from. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The electrochemical gradients are vital elements in numerous cellular processes,. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail,. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.numerade.com
SOLVED OUTSIDE ++++ ++++ ++++ ++++ INSIDE concentration gradient (with Why Is The Electrochemical Gradient Important For Cellular Function The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The importance of the electrochemical gradient is highlighted by the following points: Adenosine triphosphate, or atp, is known as the primary. To move substances against a concentration or electrochemical gradient, the cell must use energy. The precise mechanisms. Why Is The Electrochemical Gradient Important For Cellular Function.
From dokumen.tips
(PPTX) Electrochemical Gradients Ion Gradients Cell Membranes Ion Why Is The Electrochemical Gradient Important For Cellular Function The electrochemical gradients are vital elements in numerous cellular processes,. Adenosine triphosphate, or atp, is known as the primary. This energy is harvested from. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. To move substances against a concentration or electrochemical gradient, the cell must use energy.. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.numerade.com
SOLVED Fill out this chart comparing different mechanisms of transport Why Is The Electrochemical Gradient Important For Cellular Function Adenosine triphosphate, or atp, is known as the primary. Role of electrochemical gradients in cellular functions. To move substances against a concentration or electrochemical gradient, the cell must use energy. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The precise mechanisms by which a proton gradient. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.slideserve.com
PPT Membrane Structure and Function PowerPoint Presentation, free Why Is The Electrochemical Gradient Important For Cellular Function Adenosine triphosphate, or atp, is known as the primary. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy is harvested from. The importance of. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.youtube.com
Electrochemical Gradient and the Electron Transport Chain.mp4 YouTube Why Is The Electrochemical Gradient Important For Cellular Function Adenosine triphosphate, or atp, is known as the primary. Role of electrochemical gradients in cellular functions. The electrochemical gradients are vital elements in numerous cellular processes,. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The importance of the electrochemical gradient is highlighted by the following points:. Why Is The Electrochemical Gradient Important For Cellular Function.
From wou.edu
Chapter 1 The Foundations of Biochemistry Chemistry Why Is The Electrochemical Gradient Important For Cellular Function The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The importance of the electrochemical gradient is highlighted by the following points: The precise mechanisms. Why Is The Electrochemical Gradient Important For Cellular Function.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell Why Is The Electrochemical Gradient Important For Cellular Function The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The importance of the electrochemical gradient is highlighted by the following points: The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader. Why Is The Electrochemical Gradient Important For Cellular Function.
From bio.libretexts.org
8.3 Cellular Respiration Biology LibreTexts Why Is The Electrochemical Gradient Important For Cellular Function The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. Adenosine triphosphate, or atp, is known as the primary. This energy is harvested from. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important. Why Is The Electrochemical Gradient Important For Cellular Function.
From rsscience.com
Mitochondria the powerhouses of the cell definition, structure Why Is The Electrochemical Gradient Important For Cellular Function The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. Role of electrochemical gradients in cellular functions. Adenosine triphosphate, or atp, is known as the primary. This energy is harvested from. The electrochemical gradients are vital elements in numerous cellular processes,.. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.youtube.com
Electrochemical Gradients and Membrane Transport (BIOS 041) YouTube Why Is The Electrochemical Gradient Important For Cellular Function The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. Role of electrochemical gradients in cellular functions. To move substances against a concentration or electrochemical gradient, the cell must use energy. Adenosine triphosphate, or atp, is known as the primary. This energy is harvested from. The electrochemical gradient. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.slideserve.com
PPT Lecture 4 BIO 344 PowerPoint Presentation, free download ID1451459 Why Is The Electrochemical Gradient Important For Cellular Function The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. Adenosine triphosphate, or atp, is known as the primary. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells.. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.mdpi.com
Electrochem Free FullText Mass Transport Limitations in Why Is The Electrochemical Gradient Important For Cellular Function To move substances against a concentration or electrochemical gradient, the cell must use energy. Role of electrochemical gradients in cellular functions. The electrochemical gradients are vital elements in numerous cellular processes,. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. This energy is harvested from. The. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Why Is The Electrochemical Gradient Important For Cellular Function The importance of the electrochemical gradient is highlighted by the following points: The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. Adenosine triphosphate, or atp, is known as the primary. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic. Why Is The Electrochemical Gradient Important For Cellular Function.
From openbooks.lib.msu.edu
Membrane Potential Foundations of Neuroscience Why Is The Electrochemical Gradient Important For Cellular Function To move substances against a concentration or electrochemical gradient, the cell must use energy. The precise mechanisms by which a proton gradient is formed and coupled to atp synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove. Role of electrochemical gradients in cellular functions. The electrochemical gradients are vital elements in numerous cellular processes,.. Why Is The Electrochemical Gradient Important For Cellular Function.
From slideplayer.com
Membrane Structure & Function ppt download Why Is The Electrochemical Gradient Important For Cellular Function The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. Adenosine triphosphate, or atp, is known as the primary. This energy is harvested from. The. Why Is The Electrochemical Gradient Important For Cellular Function.
From courses.lumenlearning.com
The Action Potential Anatomy and Physiology I Why Is The Electrochemical Gradient Important For Cellular Function The electrochemical gradients are vital elements in numerous cellular processes,. The importance of the electrochemical gradient is highlighted by the following points: To move substances against a concentration or electrochemical gradient, the cell must use energy. The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells. Adenosine triphosphate,. Why Is The Electrochemical Gradient Important For Cellular Function.
From www.numerade.com
SOLVED The electrochemical gradient across the membrane of root cell Why Is The Electrochemical Gradient Important For Cellular Function The electrochemical gradient determines the direction that ions will flow through an open ion channel and is a combination of two types of. Adenosine triphosphate, or atp, is known as the primary. This energy is harvested from. To move substances against a concentration or electrochemical gradient, the cell must use energy. The importance of the electrochemical gradient is highlighted by. Why Is The Electrochemical Gradient Important For Cellular Function.