Electrical Ionization . the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. * ei works well for many gas phase molecules, but it does have some limitations. ion, any atom or group of atoms that bears one or more positive or negative electrical charges.
from revisionscience.com
the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. * ei works well for many gas phase molecules, but it does have some limitations. electron ionization (ei) is the most common ionization technique used for mass spectrometry. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an.
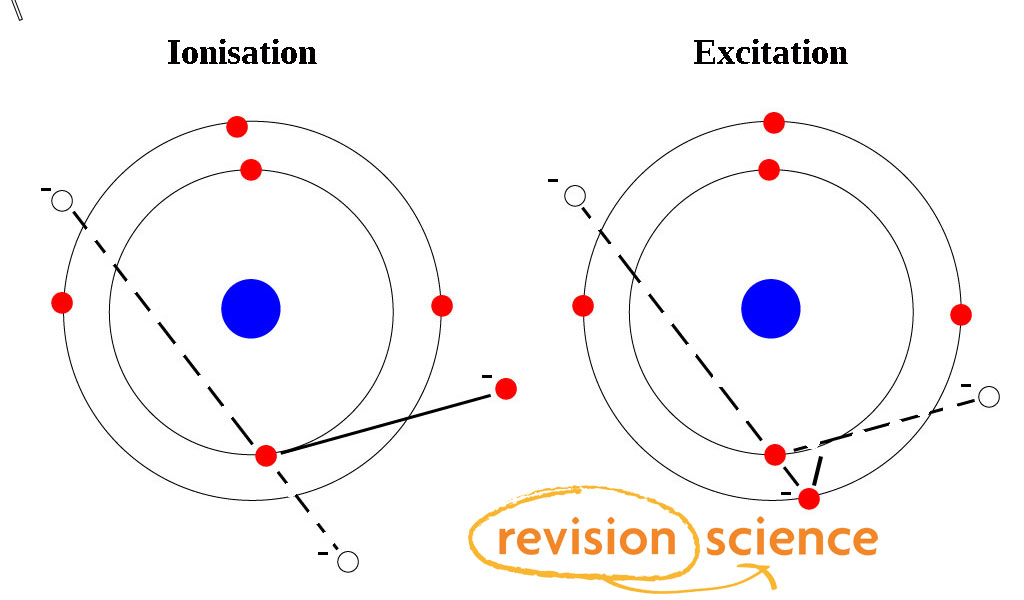
Excitation and Ionisation PhysicsALevel Revision
Electrical Ionization ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. * ei works well for many gas phase molecules, but it does have some limitations. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. electron ionization (ei) is the most common ionization technique used for mass spectrometry. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an.
From ec.bertabulle.com
How Do Ions Conduct Electricity Electrical Ionization ion, any atom or group of atoms that bears one or more positive or negative electrical charges. * ei works well for many gas phase molecules, but it does have some limitations. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. electron ionization (ei) is the most. Electrical Ionization.
From chemisfast.blogspot.com
How does ionization potential depends on atomic size nuclear charge and Electrical Ionization * ei works well for many gas phase molecules, but it does have some limitations. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ionization is a process that changes the. Electrical Ionization.
From www.researchgate.net
Field Ionization, (By presentation of Yonghai Chai, School of Chemistry Electrical Ionization ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. ionization, in. Electrical Ionization.
From www.slideserve.com
PPT Learning Objectives •Define first ionisation energy and Electrical Ionization ion, any atom or group of atoms that bears one or more positive or negative electrical charges. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. electron ionization (ei) is the most common ionization technique used for mass spectrometry. the basic form of ionization in mass spectrometry is electron. Electrical Ionization.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electrical Ionization * ei works well for many gas phase molecules, but it does have some limitations. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization is a process that changes the electrical charge of. Electrical Ionization.
From www.chemistrylearner.com
Ionization Energy Definition, Chart & Periodic Table Trend Electrical Ionization * ei works well for many gas phase molecules, but it does have some limitations. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. electron ionization (ei) is the most common ionization technique used for mass spectrometry. the basic form of ionization in mass spectrometry is electron ionization (ei), where. Electrical Ionization.
From www.researchgate.net
Electrical field lines in the ionization region of the Electrical Ionization the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization is a process that changes the electrical. Electrical Ionization.
From en.wikipedia.org
Electron ionization Wikipedia Electrical Ionization ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. * ei works well for many gas. Electrical Ionization.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend Electrical Ionization ion, any atom or group of atoms that bears one or more positive or negative electrical charges. electron ionization (ei) is the most common ionization technique used for mass spectrometry. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ionization is a process that changes the electrical charge of. Electrical Ionization.
From ionizationpandai.blogspot.com
Ionization Ionization And Electronegativity Electrical Ionization ion, any atom or group of atoms that bears one or more positive or negative electrical charges. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. * ei works well for many gas phase molecules, but it does have some limitations. the process of electron ionization (ei) yields positive radical. Electrical Ionization.
From www.alamy.com
Ionization energy (IE) Amount of energy required to remove the most Electrical Ionization ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. ionization, in chemistry and physics,. Electrical Ionization.
From therealgroupichem.weebly.com
Ionization energy Group i Chemistry(iiiescuro) Electrical Ionization the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. electron ionization (ei) is the most common ionization technique used for mass spectrometry. the process of electron ionization (ei) yields. Electrical Ionization.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Electrical Ionization ion, any atom or group of atoms that bears one or more positive or negative electrical charges. * ei works well for many gas phase molecules, but it does have some limitations. electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization, in chemistry and physics, any process by which electrically neutral atoms. Electrical Ionization.
From surfguppy.com
Ionization energy trend Surfguppy Chemistry made easy for visual Electrical Ionization * ei works well for many gas phase molecules, but it does have some limitations. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. the process of electron ionization (ei) yields. Electrical Ionization.
From www.youtube.com
Electron ionization YouTube Electrical Ionization ion, any atom or group of atoms that bears one or more positive or negative electrical charges. electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. the basic form of ionization in mass spectrometry. Electrical Ionization.
From www.slideserve.com
PPT Ionization Energy and Electron Affinity PowerPoint Presentation Electrical Ionization the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. * ei works well for many. Electrical Ionization.
From www.researchgate.net
15 Schematic diagrams showing the ionization process in both, (a Electrical Ionization the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. * ei works well for many. Electrical Ionization.
From aims.chem.utoronto.ca
Ionization AIMS Mass Spectrometry Laboratory Electrical Ionization electron ionization (ei) is the most common ionization technique used for mass spectrometry. * ei works well for many gas phase molecules, but it does have some limitations. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. the basic form of ionization in mass spectrometry is electron ionization. Electrical Ionization.
From instrumentationtools.com
Ionization chamber Principle Inst Tools Electrical Ionization electron ionization (ei) is the most common ionization technique used for mass spectrometry. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. ionization is a process that changes the electrical charge of. Electrical Ionization.
From www.researchgate.net
(a) Electrical characteristics of the ionization sensor schematically Electrical Ionization electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. * ei works well for many gas phase molecules, but it does have some limitations. ionization is a process that changes the electrical charge of atoms or molecules. Electrical Ionization.
From revisionscience.com
Excitation and Ionisation PhysicsALevel Revision Electrical Ionization ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. * ei works well for many gas phase molecules, but it does have some limitations. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. ion, any atom or group of atoms that. Electrical Ionization.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electrical Ionization the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. electron ionization (ei) is the most common ionization technique used for mass spectrometry. the basic form of ionization in mass spectrometry is electron. Electrical Ionization.
From www.radiation-dosimetry.org
What is Ionization Definition Electrical Ionization ion, any atom or group of atoms that bears one or more positive or negative electrical charges. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. ionization is a process. Electrical Ionization.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electrical Ionization the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. electron ionization (ei) is. Electrical Ionization.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend Electrical Ionization the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. the process of electron ionization (ei). Electrical Ionization.
From www.researchgate.net
Schematic of molecular ionization by external high electric field Electrical Ionization the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. ionization is a process. Electrical Ionization.
From www.electrical4u.com
Ionization Energy Electrical4U Electrical Ionization electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ionization, in chemistry and physics, any process by which. Electrical Ionization.
From www.slideserve.com
PPT IB Chemistry ATOMIC THEORY PowerPoint Presentation, free download Electrical Ionization the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. * ei works well for many gas phase molecules, but it does have some limitations. electron ionization (ei) is the most common ionization technique used for mass spectrometry. ion, any atom or group of atoms that bears one or more positive. Electrical Ionization.
From www.researchgate.net
(a) Simplified energy diagram of electron (e) field ionization, in Electrical Ionization ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. * ei works well for many gas phase molecules, but it does have some limitations. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. ionization, in chemistry and physics,. Electrical Ionization.
From www.pinterest.com
What is Ionization? First, it’s helpful to know what an ion is Electrical Ionization the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. electron ionization (ei). Electrical Ionization.
From www.shutterstock.com
Ionization Example Oxygen Process Which Neutral Stock Vector 170956625 Electrical Ionization ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. * ei works well for many gas phase molecules, but it does have some limitations. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. the basic form of ionization in mass spectrometry is electron. Electrical Ionization.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electrical Ionization ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. the process of electron ionization (ei) yields positive radical ions possessing rather high internal energy (chap. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. electron ionization (ei) is the most common. Electrical Ionization.
From www.researchgate.net
Schematic diagram of the ionization chamber. Download Scientific Diagram Electrical Ionization ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. * ei works well for many gas phase molecules, but it does have some limitations. electron ionization (ei) is the most common ionization. Electrical Ionization.
From www.researchgate.net
(A) Schematic diagram of electrospray ionization process (in positive Electrical Ionization the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. electron ionization (ei) is the most common ionization technique used for mass spectrometry. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. ionization, in chemistry and physics, any. Electrical Ionization.
From www.researchgate.net
Electric potential and ionization rate distribution on the centerline Electrical Ionization electron ionization (ei) is the most common ionization technique used for mass spectrometry. the basic form of ionization in mass spectrometry is electron ionization (ei), where an electron beam, usually at an. ionization is a process that changes the electrical charge of atoms or molecules by gaining or losing electrons. * ei works well for many gas. Electrical Ionization.