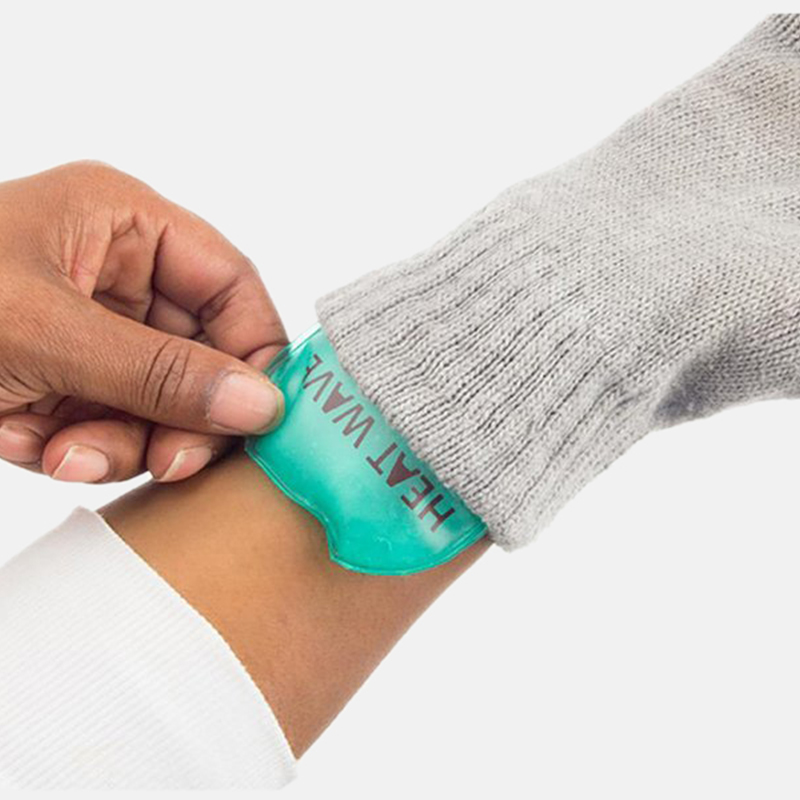
Reusable Hand Warmer (Nucleation) . To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. this is a sodium acetate reusable hand warmer. They have a supersaturated sodium acetate solution inside the packet. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The flexing of the small metal disc initiates nucleation in the. By pressing a button in a pouch, which. As the solution crystalizes (sodium being salt), it releases energy as heat. reusable hand warmers work a little differently.
from www.tanga.com
They have a supersaturated sodium acetate solution inside the packet. The flexing of the small metal disc initiates nucleation in the. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. By pressing a button in a pouch, which. reusable hand warmers work a little differently. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. As the solution crystalizes (sodium being salt), it releases energy as heat. this is a sodium acetate reusable hand warmer.
2pk Heat Wave Instant Reusable Hand Warmers Tanga
Reusable Hand Warmer (Nucleation) They have a supersaturated sodium acetate solution inside the packet. By pressing a button in a pouch, which. To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. reusable hand warmers work a little differently. this is a sodium acetate reusable hand warmer. The flexing of the small metal disc initiates nucleation in the. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. They have a supersaturated sodium acetate solution inside the packet. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. As the solution crystalizes (sodium being salt), it releases energy as heat.
From www.desertcart.ae
Buy Reusable Heat Packs, Set of 8 Gel Hand Warmer with Snap to Heat Reusable Hand Warmer (Nucleation) hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. By pressing a button in a pouch, which. To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. reusable hand warmers work a little differently. They have a supersaturated sodium acetate solution. Reusable Hand Warmer (Nucleation).
From www.groupon.com
Reusable Hand Warmers Groupon Goods Reusable Hand Warmer (Nucleation) reusable hand warmers work a little differently. As the solution crystalizes (sodium being salt), it releases energy as heat. By pressing a button in a pouch, which. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. this is a sodium acetate reusable hand warmer. hand warmers provide a unique. Reusable Hand Warmer (Nucleation).
From www.outdoorhub.com
Keep Warm with The Best Reusable Hand Warmers OutdoorHub Reusable Hand Warmer (Nucleation) reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. By pressing a button in a pouch, which. reusable hand warmers work a little differently. The flexing of the small metal disc initiates. Reusable Hand Warmer (Nucleation).
From www.ecoloco.ca
Reusable hand warmers Eco Loco Reusable Hand Warmer (Nucleation) To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. this is a sodium acetate reusable hand warmer. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. As the solution crystalizes (sodium being salt), it releases energy as heat. They have. Reusable Hand Warmer (Nucleation).
From www.thegreatoutdoorsmag.com
Lifesystems Reusable Hand Warmers review TGO Magazine Reusable Hand Warmer (Nucleation) this is a sodium acetate reusable hand warmer. reusable hand warmers work a little differently. They have a supersaturated sodium acetate solution inside the packet. To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. The flexing of the small metal disc initiates nucleation in the. By pressing a button. Reusable Hand Warmer (Nucleation).
From www.walmart.com
Gel Hand Warmer 2 Pack Reusable Heating Pads for Cold Hands Reusable Hand Warmer (Nucleation) The flexing of the small metal disc initiates nucleation in the. As the solution crystalizes (sodium being salt), it releases energy as heat. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. reusable hand warmers work a little differently. this is a sodium acetate reusable hand warmer. To. Reusable Hand Warmer (Nucleation).
From www.pproreviews.com
Top 10 Best Rechargeable Hand Warmers in 2024 Reviews Reusable Hand Warmer (Nucleation) reusable hand warmers work a little differently. By pressing a button in a pouch, which. As the solution crystalizes (sodium being salt), it releases energy as heat. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. this is a sodium acetate reusable hand warmer. They have a supersaturated. Reusable Hand Warmer (Nucleation).
From www.leevalley.com
Reusable Instant Hand Warmer Lee Valley Tools Reusable Hand Warmer (Nucleation) They have a supersaturated sodium acetate solution inside the packet. reusable hand warmers work a little differently. As the solution crystalizes (sodium being salt), it releases energy as heat. By pressing a button in a pouch, which. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The flexing of. Reusable Hand Warmer (Nucleation).
From www.walmart.ca
Perfect Solutions Reusable Hand Warmers (set of 2) Walmart Canada Reusable Hand Warmer (Nucleation) They have a supersaturated sodium acetate solution inside the packet. reusable hand warmers work a little differently. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. As the solution crystalizes (sodium being salt), it releases energy as heat. To reuse these hand warmers, boil the closed packet in water. Reusable Hand Warmer (Nucleation).
From www.brookstone.com
Insta Heat Reusable Hand Warmers 10 Pack Brookstone Reusable Hand Warmer (Nucleation) this is a sodium acetate reusable hand warmer. They have a supersaturated sodium acetate solution inside the packet. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. As the solution crystalizes (sodium being salt), it releases energy as heat. To reuse these hand warmers, boil the closed packet in water to. Reusable Hand Warmer (Nucleation).
From www.youtube.com
Activating and Recharging the UST Reusable Hand Warmer YouTube Reusable Hand Warmer (Nucleation) As the solution crystalizes (sodium being salt), it releases energy as heat. To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. reusable hand warmers work a little differently. this is a sodium acetate reusable hand warmer. reusable handwarmers are made of something dissolved nearly to its saturation point. Reusable Hand Warmer (Nucleation).
From survivaldefensestore.com
ReDo Reusable Rechargeable Hand Warmers 2Pack Reusable Hand Warmer (Nucleation) hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. this is a sodium acetate reusable hand warmer. reusable hand warmers work a little differently. As the solution crystalizes (sodium being salt), it releases energy as heat. By pressing a button in a pouch, which. To reuse these hand. Reusable Hand Warmer (Nucleation).
From www.alamy.com
Reusable hand warmer hires stock photography and images Alamy Reusable Hand Warmer (Nucleation) To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. this is a sodium acetate reusable hand warmer. As the solution crystalizes (sodium being salt), it releases energy as heat. The flexing of the small metal disc initiates nucleation in the. hand warmers provide a unique and fun way to. Reusable Hand Warmer (Nucleation).
From www.heatpad.cn
Reusable Instant Click Heat Hand Warmer Heating Pads Relieve Back Reusable Hand Warmer (Nucleation) reusable hand warmers work a little differently. To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. As the solution crystalizes (sodium being salt), it releases energy as heat. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. They have a. Reusable Hand Warmer (Nucleation).
From hotsnapz.com
HotSnapZ Reusable Hand Warmers and Heat Pads Reusable Hand Warmer (Nucleation) hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. As the solution crystalizes (sodium being salt), it releases energy as heat. The flexing of the small metal disc initiates nucleation in the. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. They. Reusable Hand Warmer (Nucleation).
From www.westmarine.com
ULTIMATE SURVIVAL TECHNOLOGIES Reusable Hand Warmer, 4Pack West Marine Reusable Hand Warmer (Nucleation) By pressing a button in a pouch, which. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. As the solution crystalizes (sodium being salt), it releases energy as heat. They have a supersaturated sodium acetate solution inside the packet. The flexing of the small metal disc initiates nucleation in the. To reuse. Reusable Hand Warmer (Nucleation).
From plasticfreereviews.com
How do reusable Hand Warmers Work Plastic Free Reviews Reusable Hand Warmer (Nucleation) They have a supersaturated sodium acetate solution inside the packet. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. reusable hand warmers work a little differently. The flexing of the small metal disc initiates nucleation in the. To reuse these hand warmers, boil the closed packet in water to return the. Reusable Hand Warmer (Nucleation).
From www.tanga.com
2Pack Heat Wave Instant Reusable Hand Warmers Tanga Reusable Hand Warmer (Nucleation) hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. The flexing of the small metal disc initiates nucleation in the. They have a supersaturated sodium acetate solution inside the packet. By pressing. Reusable Hand Warmer (Nucleation).
From www.bitsandpieces.com
Shop FastActing Reusable Hand Warmers Set of 2 Reusable Hand Warmer (Nucleation) reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. The flexing of the small metal disc initiates nucleation in the. They have a supersaturated sodium acetate solution inside the packet. As the solution crystalizes (sodium being salt), it releases energy as heat. reusable hand warmers work a little differently. By pressing. Reusable Hand Warmer (Nucleation).
From survivaldefensestore.com
ReDo Reusable Rechargeable Hand Warmers 2Pack Reusable Hand Warmer (Nucleation) reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. this is a sodium acetate reusable hand warmer. reusable hand warmers work a little differently. They have a supersaturated sodium acetate solution inside the packet. The flexing of the small metal disc initiates nucleation in the. hand warmers provide a. Reusable Hand Warmer (Nucleation).
From www.pinterest.com
Reusable Hand Warmer Via Exothermic Crystallization Reusable hand Reusable Hand Warmer (Nucleation) this is a sodium acetate reusable hand warmer. By pressing a button in a pouch, which. They have a supersaturated sodium acetate solution inside the packet. As the solution crystalizes (sodium being salt), it releases energy as heat. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The flexing. Reusable Hand Warmer (Nucleation).
From www.amazon.ca
Hot Poc Reusable Hand Warmers, Sustainable, No Charge Needed, Pocket Reusable Hand Warmer (Nucleation) They have a supersaturated sodium acetate solution inside the packet. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The flexing of the small metal disc initiates nucleation in the. reusable hand warmers work a little differently. By pressing a button in a pouch, which. this is a. Reusable Hand Warmer (Nucleation).
From www.ebay.com
Pack 2 Instant Heating GEL HAND WARMERS Reusable Heat Packs Pads Reusable Hand Warmer (Nucleation) reusable hand warmers work a little differently. By pressing a button in a pouch, which. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. this is a sodium acetate reusable hand warmer. To reuse these hand warmers, boil the closed packet in water to return the crystals to. Reusable Hand Warmer (Nucleation).
From www.youtube.com
Reusable Hand Warmer (Nucleation) YouTube Reusable Hand Warmer (Nucleation) this is a sodium acetate reusable hand warmer. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. As the solution crystalizes (sodium being salt), it releases energy as heat. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. They have a. Reusable Hand Warmer (Nucleation).
From www.amazon.com
Proheat Reusable Hand Warmer Health & Household Reusable Hand Warmer (Nucleation) reusable hand warmers work a little differently. By pressing a button in a pouch, which. The flexing of the small metal disc initiates nucleation in the. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. hand warmers provide a unique and fun way to study the chemistry of crystal formation. Reusable Hand Warmer (Nucleation).
From www.naturkompaniet.se
Life Systems REUSABLE HAND WARMERS Handvärmare Naturkompaniet Reusable Hand Warmer (Nucleation) reusable hand warmers work a little differently. The flexing of the small metal disc initiates nucleation in the. They have a supersaturated sodium acetate solution inside the packet. this is a sodium acetate reusable hand warmer. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. To reuse these hand warmers,. Reusable Hand Warmer (Nucleation).
From www.naturkompaniet.se
Life Systems REUSABLE HAND WARMERS Handvärmare Naturkompaniet Reusable Hand Warmer (Nucleation) hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. reusable hand warmers work a little differently. this is a sodium acetate reusable hand warmer. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. They have a supersaturated sodium acetate solution. Reusable Hand Warmer (Nucleation).
From catstycam.com
Lifesystems Reusable Handwarmers 2 Pack Reusable Hand Warmer (Nucleation) reusable hand warmers work a little differently. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. this is a sodium acetate reusable hand warmer. The flexing of the small metal disc initiates. Reusable Hand Warmer (Nucleation).
From plasticfreereviews.com
How do reusable Hand Warmers Work Plastic Free Reviews Reusable Hand Warmer (Nucleation) As the solution crystalizes (sodium being salt), it releases energy as heat. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. reusable hand warmers work a little differently. They have a supersaturated sodium acetate solution inside the packet. reusable handwarmers are made of something dissolved nearly to its. Reusable Hand Warmer (Nucleation).
From www.ellis-brigham.com
Lifesystems Reusable Hand Warmers Ellis Brigham Reusable Hand Warmer (Nucleation) As the solution crystalizes (sodium being salt), it releases energy as heat. reusable hand warmers work a little differently. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. They have a supersaturated sodium acetate solution inside the packet. reusable handwarmers are made of something dissolved nearly to its. Reusable Hand Warmer (Nucleation).
From spy.com
The Best Reusable Hand Warmers for Winter 2023 Reusable Hand Warmer (Nucleation) reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. They have a supersaturated sodium acetate solution inside the packet. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The flexing of the small metal disc initiates nucleation in the. As the solution. Reusable Hand Warmer (Nucleation).
From generise.co.uk
Generise Reusable Hand Warmer with Knitted Cover Single or Double Ra Reusable Hand Warmer (Nucleation) They have a supersaturated sodium acetate solution inside the packet. The flexing of the small metal disc initiates nucleation in the. reusable hand warmers work a little differently. By pressing a button in a pouch, which. hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. To reuse these hand. Reusable Hand Warmer (Nucleation).
From www.tanga.com
2pk Heat Wave Instant Reusable Hand Warmers Tanga Reusable Hand Warmer (Nucleation) reusable hand warmers work a little differently. By pressing a button in a pouch, which. The flexing of the small metal disc initiates nucleation in the. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. To reuse these hand warmers, boil the closed packet in water to return the crystals to. Reusable Hand Warmer (Nucleation).
From www.naturkompaniet.se
Life Systems REUSABLE HAND WARMERS Handvärmare Naturkompaniet Reusable Hand Warmer (Nucleation) To reuse these hand warmers, boil the closed packet in water to return the crystals to a liquid. this is a sodium acetate reusable hand warmer. reusable hand warmers work a little differently. By pressing a button in a pouch, which. hand warmers provide a unique and fun way to study the chemistry of crystal formation and. Reusable Hand Warmer (Nucleation).
From www.walmart.com
Beaut Instant Hot Spots Reusable Hand Warmers 6 Pack Small Packs Reusable Hand Warmer (Nucleation) this is a sodium acetate reusable hand warmer. As the solution crystalizes (sodium being salt), it releases energy as heat. reusable handwarmers are made of something dissolved nearly to its saturation point in hot water (sodium. reusable hand warmers work a little differently. They have a supersaturated sodium acetate solution inside the packet. hand warmers provide. Reusable Hand Warmer (Nucleation).