Ap Chemistry Buffer Frqs . Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. (i) calculate the value of [h 3 o+] in the buffer solution. The second student or the buffer made by the first student in part (c) ? Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. The ph of the buffer solution is determined to be 6.48. Exam ordering, administration & scores. Ph when a strong acid or a strong base is added, the buffer made by. A buffer resists a change in ph.
from www.studocu.com
Exam ordering, administration & scores. The ph of the buffer solution is determined to be 6.48. The second student or the buffer made by the first student in part (c) ? Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. (i) calculate the value of [h 3 o+] in the buffer solution. Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. Ph when a strong acid or a strong base is added, the buffer made by. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. A buffer resists a change in ph. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium.
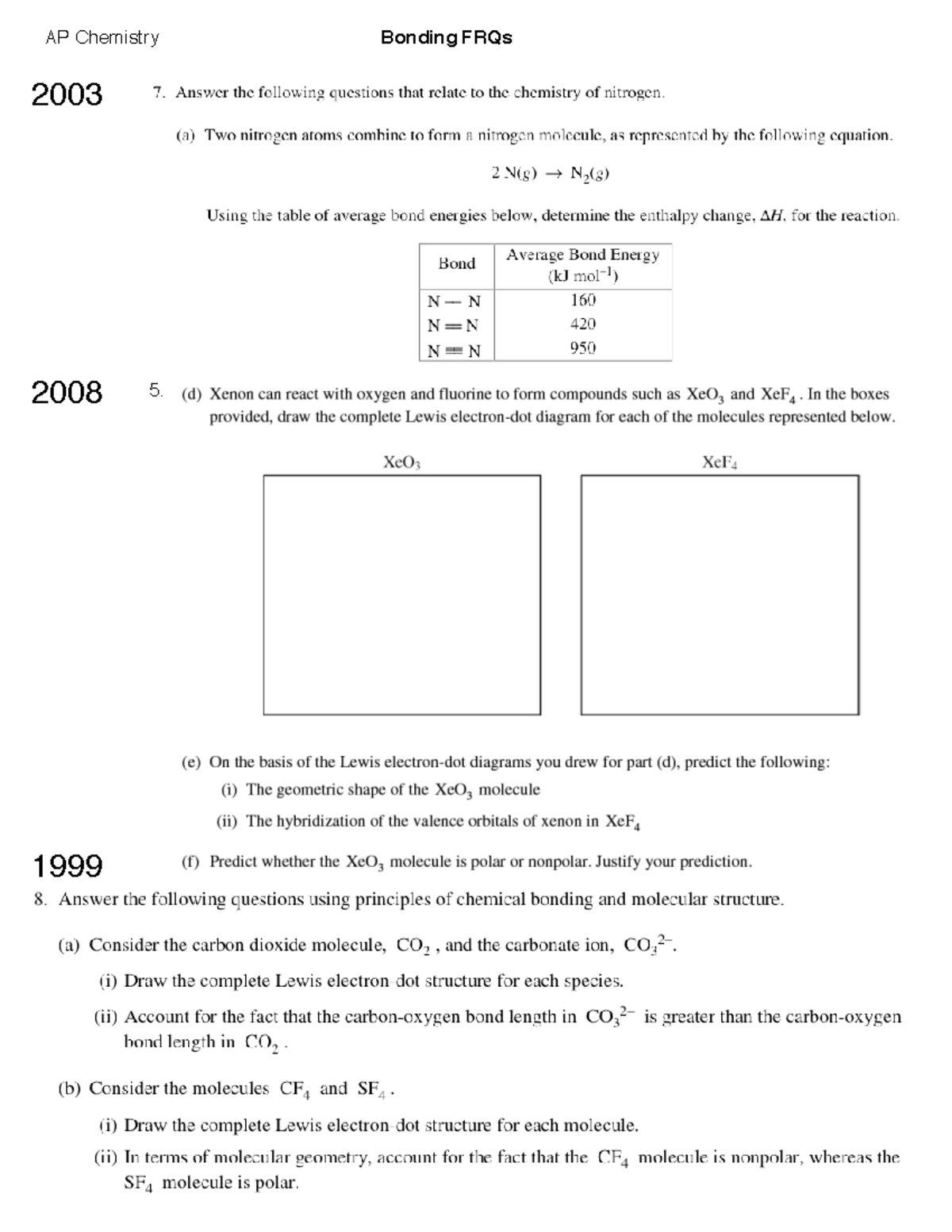
08Bonding FRQs bonding frqs AP Chemistry Bonding FRQs 2003 2008 5
Ap Chemistry Buffer Frqs Exam ordering, administration & scores. Ph when a strong acid or a strong base is added, the buffer made by. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. The ph of the buffer solution is determined to be 6.48. Exam ordering, administration & scores. (i) calculate the value of [h 3 o+] in the buffer solution. The second student or the buffer made by the first student in part (c) ? Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. A buffer resists a change in ph.
From dokumen.tips
(PDF) AP Chemistry Acid/Base FRQs 1999 2000 the data from the titration Ap Chemistry Buffer Frqs Exam ordering, administration & scores. Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. A buffer resists a change in ph. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. (i) calculate the value of [h 3 o+] in the. Ap Chemistry Buffer Frqs.
From www.edvotek.com
AP® Chemistry Lab 16 Preparation of Effective Buffers Kit EDVOTEK Ap Chemistry Buffer Frqs Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. Ph when a strong acid or a strong base is added, the buffer made by. Since a buffer. Ap Chemistry Buffer Frqs.
From www.showme.com
ap chemistry buffers Science, buffers ShowMe Ap Chemistry Buffer Frqs The ph of the buffer solution is determined to be 6.48. The second student or the buffer made by the first student in part (c) ? (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Ph when a strong acid or a strong base is added, the buffer made by.. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chem Common Ion Effect and Buffers 1 YouTube Ap Chemistry Buffer Frqs (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. The ph of the buffer solution is determined to be 6.48. Questions and model answers on buffers for the college board ap®. Ap Chemistry Buffer Frqs.
From studylib.net
AP Chem Unit 1 FRQ practice Ap Chemistry Buffer Frqs The ph of the buffer solution is determined to be 6.48. Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Ph when a strong acid or a strong base is added, the buffer made by. The second student or the buffer made by the first student in. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chemistry Buffers and HendersonHasselbalch Equation YouTube Ap Chemistry Buffer Frqs (i) calculate the value of [h 3 o+] in the buffer solution. Ph when a strong acid or a strong base is added, the buffer made by. Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Since a buffer consists of both an acid or base and. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chemistry, Unit 7 Equilibrium Notes Part 5 YouTube Ap Chemistry Buffer Frqs The second student or the buffer made by the first student in part (c) ? The ph of the buffer solution is determined to be 6.48. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. (i) calculate the value of [h 3 o+] in the buffer solution. Given a solution. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chem Buffers YouTube Ap Chemistry Buffer Frqs Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. A buffer resists a change in ph. The ph of the buffer solution is determined to be 6.48. (i). Ap Chemistry Buffer Frqs.
From www.flinnsci.ca
FlinnPREP™ Inquiry Labs for AP® Chemistry Buffers in Household Ap Chemistry Buffer Frqs (i) calculate the value of [h 3 o+] in the buffer solution. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. The second student or the buffer made by the first student in part (c) ? Given a solution of ammonium chloride, what additional reagent or reagents are needed to. Ap Chemistry Buffer Frqs.
From www.youtube.com
What You Need to Know About Buffers AP Chem Unit 8, Topics 810 YouTube Ap Chemistry Buffer Frqs Exam ordering, administration & scores. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. Ph when a strong acid or a strong base is added, the buffer made by. A buffer resists a change in ph. Questions and model answers on buffers for the college board ap® chemistry syllabus,. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chem 4.3 buffer solutions YouTube Ap Chemistry Buffer Frqs Exam ordering, administration & scores. The second student or the buffer made by the first student in part (c) ? A buffer resists a change in ph. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. Questions and model answers on buffers for the college board ap® chemistry syllabus,. Ap Chemistry Buffer Frqs.
From kelsiyyolanthe.pages.dev
Ap Chem 2024 Frq Scoring Guidelines 2024 Trude Hortense Ap Chemistry Buffer Frqs (i) calculate the value of [h 3 o+] in the buffer solution. Ph when a strong acid or a strong base is added, the buffer made by. Exam ordering, administration & scores. A buffer resists a change in ph. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Given a. Ap Chemistry Buffer Frqs.
From www.chegg.com
Solved Page 1 AP Chem worksheetBuffers, The common ion Ap Chemistry Buffer Frqs (i) calculate the value of [h 3 o+] in the buffer solution. Exam ordering, administration & scores. The second student or the buffer made by the first student in part (c) ? Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. The ph of the buffer solution is determined. Ap Chemistry Buffer Frqs.
From dokumen.tips
(PDF) AP Chemistry Experiment FRQs · PDF fileAP Chemistry Experiment Ap Chemistry Buffer Frqs (i) calculate the value of [h 3 o+] in the buffer solution. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Ph when a strong acid or a strong base is added, the buffer made by. A buffer resists a change in ph. The second student or the buffer made. Ap Chemistry Buffer Frqs.
From www.studocu.com
08Bonding FRQs bonding frqs AP Chemistry Bonding FRQs 2003 2008 5 Ap Chemistry Buffer Frqs The second student or the buffer made by the first student in part (c) ? A buffer resists a change in ph. Ph when a strong acid or a strong base is added, the buffer made by. Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Given. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chem Buffers Part I YouTube Ap Chemistry Buffer Frqs Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. The second student or the buffer made by the first student in part (c) ? (d) a buffer. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chem Unit 8.6 Buffers YouTube Ap Chemistry Buffer Frqs The second student or the buffer made by the first student in part (c) ? Ph when a strong acid or a strong base is added, the buffer made by. The ph of the buffer solution is determined to be 6.48. A buffer resists a change in ph. (i) calculate the value of [h 3 o+] in the buffer solution.. Ap Chemistry Buffer Frqs.
From studylib.net
Aqueous Equilibria Buffers & Titrations AMHS AP Chemistry Ap Chemistry Buffer Frqs Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. The second student or the buffer made by the first student in part (c) ? (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. The ph of the buffer solution is. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chem U8 Buffer Calculations Using HendersonHasselbalch Equation Ap Chemistry Buffer Frqs The second student or the buffer made by the first student in part (c) ? (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. The ph of the buffer solution. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP chem 10.4 buffers YouTube Ap Chemistry Buffer Frqs Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. Exam ordering, administration & scores. The second student or the buffer made by the first student in part (c) ? Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save. Ap Chemistry Buffer Frqs.
From www.studocu.com
Acid Base Buffers Homework Part C 1 AP Chemistry Acid Base Buffers Ap Chemistry Buffer Frqs (i) calculate the value of [h 3 o+] in the buffer solution. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. The ph of the buffer solution is determined to be 6.48. The second student or the buffer made by the first student in part (c) ? Ph when a. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP CHEMISTRY Unit 8 Buffers YouTube Ap Chemistry Buffer Frqs The ph of the buffer solution is determined to be 6.48. The second student or the buffer made by the first student in part (c) ? Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Ph when a strong acid or a strong base is added, the. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chemistry Buffers YouTube Ap Chemistry Buffer Frqs Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. Ph when a strong acid or a strong base is added, the buffer made by. A buffer resists a. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chem U3 Solubility FRQs and Separation Techniques YouTube Ap Chemistry Buffer Frqs Ph when a strong acid or a strong base is added, the buffer made by. A buffer resists a change in ph. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. The ph of the buffer solution is determined to be 6.48. (i) calculate the value of [h 3. Ap Chemistry Buffer Frqs.
From quizlet.com
AP Chem Buffers Test Diagram Quizlet Ap Chemistry Buffer Frqs A buffer resists a change in ph. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. The second student or the buffer made by the first student in part (c) ? Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts. Ap Chemistry Buffer Frqs.
From www.youtube.com
2012 AP Chem FRQs 2 3 4 YouTube Ap Chemistry Buffer Frqs Ph when a strong acid or a strong base is added, the buffer made by. A buffer resists a change in ph. The second student or the buffer made by the first student in part (c) ? (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Questions and model answers. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chem Unit 5 PC FRQ Key YouTube Ap Chemistry Buffer Frqs Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. The ph of the buffer solution is determined to be 6.48. Ph when a strong acid or a strong base is. Ap Chemistry Buffer Frqs.
From www.pinterest.com.au
Buffer solutions Buffer solution, Chemistry lessons, Ap chemistry Ap Chemistry Buffer Frqs Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. Ph when a strong acid or a strong base is added, the buffer made by. The second student or the buffer made by the first student in part (c) ? The ph of the buffer solution is determined to be 6.48.. Ap Chemistry Buffer Frqs.
From www.studocu.com
15 Buffers MADE EASY AP CHEM REVIEW AP* Chemistry Buffers Made Easy Ap Chemistry Buffer Frqs (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the. Ap Chemistry Buffer Frqs.
From studylib.net
Buffer Equilibrium Free Response Questions Ap Chemistry Buffer Frqs Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. Exam ordering, administration & scores. The ph of the buffer solution is determined to be 6.48. Ph when a strong acid or a strong base is added, the buffer made by. Given a solution of ammonium chloride, what additional reagent or. Ap Chemistry Buffer Frqs.
From www.youtube.com
AP Chemistry review of buffer question YouTube Ap Chemistry Buffer Frqs Ph when a strong acid or a strong base is added, the buffer made by. Exam ordering, administration & scores. Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my. Ap Chemistry Buffer Frqs.
From www.youtube.com
Buffer capacity Acids and bases AP Chemistry Khan Academy YouTube Ap Chemistry Buffer Frqs (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my. Ap Chemistry Buffer Frqs.
From slideplayer.com
Buffers AP Chemistry. ppt video online download Ap Chemistry Buffer Frqs (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Exam ordering, administration & scores. Questions and model answers on buffers for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. A buffer resists a change in ph. Since a buffer consists of both an. Ap Chemistry Buffer Frqs.
From www.youtube.com
Properties of buffers Acids and bases AP Chemistry Khan Academy Ap Chemistry Buffer Frqs Since a buffer consists of both an acid or base and its conjugate, which differ by an h +,. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. Given a solution of ammonium chloride, what additional reagent or reagents are needed to prepare a buffer from the ammonium. Questions and. Ap Chemistry Buffer Frqs.
From www.youtube.com
Methods for preparing buffers Acids and bases AP Chemistry Khan Ap Chemistry Buffer Frqs Exam ordering, administration & scores. (d) a buffer solution is prepared by dissolving some solid naocl in a solution of hocl at 298 k. The ph of the buffer solution is determined to be 6.48. Ph when a strong acid or a strong base is added, the buffer made by. Since a buffer consists of both an acid or base. Ap Chemistry Buffer Frqs.